1. Background
Breast cancer (BC) is one of the main causes of cancer-related death in women, worldwide (1, 2). It is known that a combination of various factors, including genetic mutations, hormones, and physical, chemical, and environmental factors are involved in the pathogenesis of BC (3-5). Inherited mutations in BRCA1 and BRCA2 tumor suppressor genes, for instance, are associated with high-risk BC and ovarian cancer development in young women (6). In addition, several other molecules and aberrant signaling pathways have been implicated in metastasis and invasion of cancer (7), though much remains to be known in the context of BC.
Motility-related protein-1 (MRP-1/CD9) is a member of tetraspanins that interacts with a variety of transmembrane proteins and other cell-surface molecules (8-10). It has been implicated in various biological functions, such as cell-cell fusion, adhesion, cell migration, proliferation, differentiation, and survival (9, 11, 12). CD9 was primarily identified as a suppressor of metastasis in cancer (10, 12). Down-regulation of CD9 is associated with poor prognosis in a variety of cancers, including multiple melanomas (13), non-small-cell lung cancer (14), colon (15), breast (16), ovarian (17), and prostate cancers (18). On the other hand, recent studies on human BC cell lines have suggested a metastasis-promoting role for CD9 (19, 20). However, this hypothesis remains to be validated in human breast tumors.
Matrix metalloproteinases (MMPs) belong to a family of zinc-dependent endopeptidases involved not only in remodeling the extracellular matrix during development and tissue homeostasis but also implicated in inflammation and cancer progression via promoting angiogenesis and metastasis (21). Matrix metalloproteinase 9 or gelatinase B is a collagenase that degrades type IV collagen, a main component of the basement membrane, as well as gelatin and fibronectin (22). There is an increasing line of evidence that overexpression of MMP9 is associated with invasion, metastasis, and poor prognosis in different types of human cancers including BC (23-25).
2. Objectives
Considering the MMP9 as one of the main drivers of metastasis in human solid tumors, herein we aimed simultaneously at analyzing the expression of both CD9 and MMP9 and their possible association in human BC tumors from Iranian women to further provide evidence regarding tumor suppressor or promoting activity of CD9.
3. Methods
3.1. Study Population and Samples
A total of 24 samples were obtained, including 19 breast tissues from women with BC and 5 normal breast tissues from volunteer healthy women referring to Khatam Ol Anbia Specialty and Subspecialty Hospital of Tehran, Tehran, Iran. The normal samples were obtained during the mammoplasty of breasts with no evidence of abnormalities such as carcinoma or adenoma. Clinical information and demographic characteristics of the participant were collected. The presence of neoplastic cells was the feature of BC detection by the pathologist. Tumors were examined for estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor (HER), P53, and KI-67. All patients signed informed consent. Also, the study procedure was approved by the ethical committee, based at the university, regarding patients’ awareness and study compliance with ethical issues.
3.2. RNA Extraction
The total RNA was extracted, using RNX PLUS total RNA isolation reagent (SinaClon; Iran) according to the manufacturer’s instructions. Briefly, the frozen tissues were mechanically homogenized, using 1 mL RNX PLUS in 1.5 mL centrifuge tubes and incubated at room temperature for 5 min. Next, 200 μL chloroform was added and incubated on ice for 5 min, followed by centrifugation at 12000 rpm, 4°C for 15 min. Subsequently, the aqueous phase transferred to new RNase free-DNase free and RNA content was precipitated by the addition of 500 μL isopropanol and incubating on ice for 15 min, followed by centrifugation at 12000 rpm, 4°C for 15 min. After removal of the supernatant, the pelleted was washed, using 1 mL of ethanol and centrifugation at 75000 rpm, 4°C for 8 min. Finally, concentrated RNA was eluted in 25 μL volumes of nuclease-free water.
3.3. Complementary DNA Synthesis
The mRNA content was converted to complementary DNA (cDNA), using Reverse Transcription (RT) Kit following the manufacturer’s protocols (Maxime RT Premix, iNtRON Biotechnology, South Korea). Briefly, the total RNA (7 μL) and nuclease-free water (13 μL) were added to premix 200-μL tubes, which contained all components for polymerase chain reaction (PCR) except RNA and water. The RT reaction was performed in a final volume of 20 μL at 45°C for 1 hour, followed by 95°C for 5 min (to inactivate of RTase).
3.4. Quantitative Polymerase Chain Reaction (qPCR) Assay
Expression of MMP9 and CD9 (target genes) and β2M (reference gene) were quantified, using a Real-Time PCR System thermal cycler (Illumina), SYBR® Green Master Mix-Plus (Ampliqon, Odense, Denmark) in a total volume of 10 μL by using specific primers as follows: MMP9 forward, 5’-GCAGGATGTCATAGGTCACG-3’; and MMP9 reverse, 5’-TCCAGTACCGAGAGAAAGCC-3’; and CD9 forward, 5’-TCCAGCTTCTACACAGGAGTC-3’; and CD9 reverse, 5’-AGGAAGCCGAAGAACAGTC-3; β2m forward, 5’-CTACTCTCTCTTTCTGGCCTG-3; and β2m reverse, 5’-GACAAGTCTGAATGCTCCAC -3’. The reaction program included activation at 95°C for 15 min, followed by 45 cycles of 3 steps of denaturation at 95°C for 25 s, annealing at 62°C for 30 s, and extension at 72°C for 20 s. All reactions were performed in duplicate in a 48-well optical plate.
3.5. Statistical Analysis
The statistical analysis was performed, using GraphPad Prism5 for Windows (GraphPad Software, Inc., La Jolla, CA, USA). Any differences in expression values between groups were examined, using Mann-Whitney and Kruskal Wallis tests. The association between the expressions of MM9 and CD9 was assessed by the Pearson correlation test. The threshold to indicate a statistically significant difference was P ≤ 0.05.
4. Results
The demographic and clinical characteristics of the participants are shown in Table 1. All the patients were females with a mean age of 58.4 2 ± 12.47 years (range, 34 - 74 years). The mean age of the control group was 52.40 ± 5.77 years (range, 44 - 58 years). There was no significant difference between the patients and the control group regarding the mean age (P = 0.139). Concerning the pathology of the disease, 17 cases (89.5%) presented invasive ductal carcinoma (IDC), 1 case (5.25%) medullary carcinoma (MC), and 1 case (5.25%) invasive lobular carcinoma (ILC).
| Characteristics | Patient | Control |
|---|---|---|
| N | 19 | 5 |
| Age (y) | 58.42 ± 12.47 | 52.40 ± 5.77 |
| Tumor size (cm) | 3.01 ± 0.797 | N/A |
| Pathology type | ||
| Invasive ductal carcinoma | 17 (89.5) | N/A |
| Invasive lobular carcinoma | 1 (5.3) | N/A |
| Medullary carcinoma | 1 (5.3) | N/A |
| Estrogen receptor | ||
| Positive | 14 (73.7) | N/A |
| Negative | 5 (26.3) | N/A |
| Progesterone receptor | ||
| Positive | 14 (73.7) | N/A |
| Negative | 5 (26.3) | N/A |
| Human epidermal growth factor receptor 2 | ||
| Positive | 4 (20.1) | N/A |
| Negative | 15 (79.9) | N/A |
| P53 | ||
| Positive | 5 (26.3) | N/A |
| Negative | 14 (73.7) | N/A |
| KI-67 | ||
| Positive | 18 (94.7) | N/A |
| Negative | 1 (5.3) | N/A |
a Values are expressed as mean ± SD or No. (%).
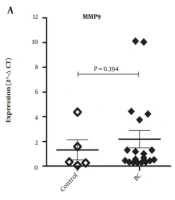
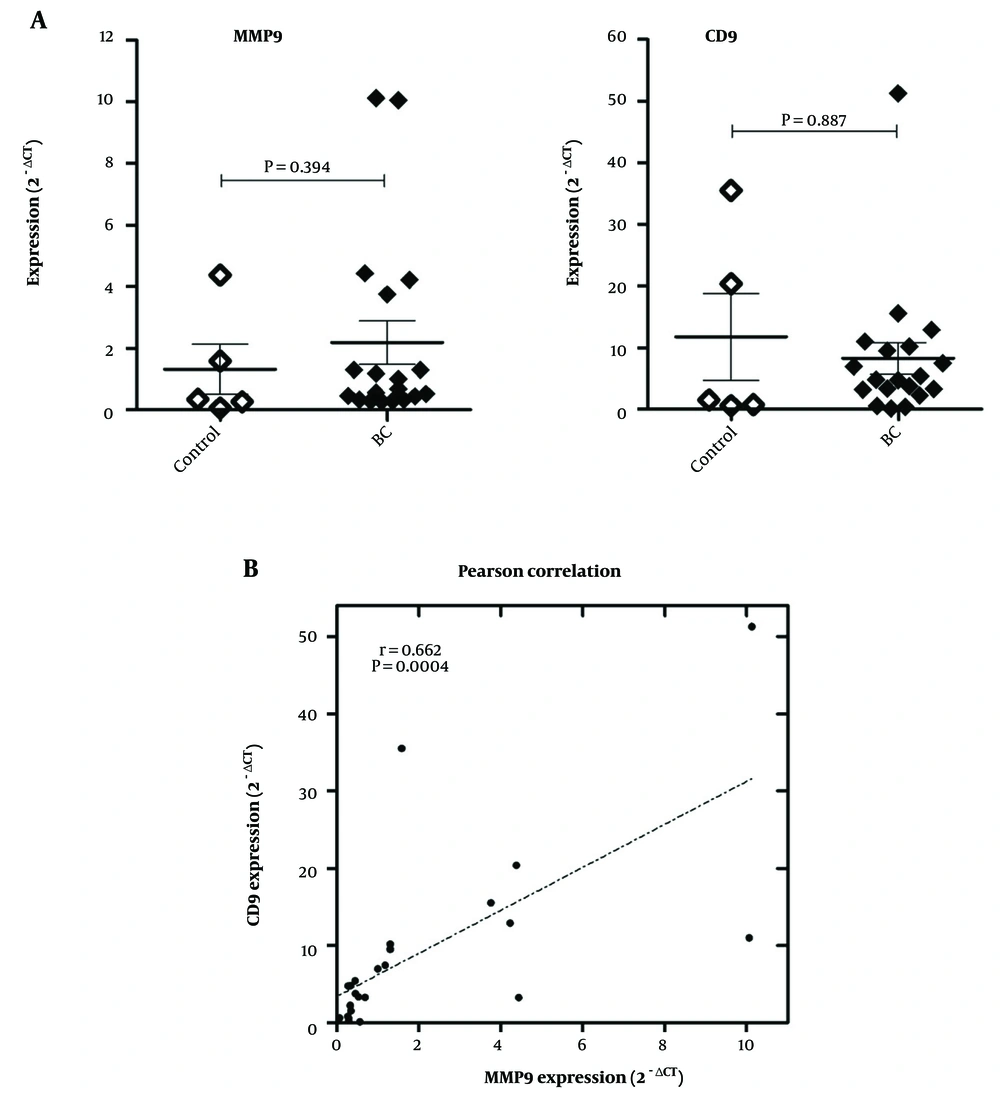
The mRNA expressions of MMP9 and CD9 were analyzed in breast carcinoma and normal breast tissues. As depicted in Figure 1A. There was no statistically significant difference between BC and the control group regarding the mean tissue expression of MMP9 (P = 0.394) and CD9 (P = 0.887). However, interestingly, a significant strong positive correlation was observed between CD9 and MMP-9 expressions (Figure 1B; r = 0.761 and P = 0.0002).
A, Comparative expression of matrix metalloproteinase 9 (MMP9) and motility-related protein-1 (MRP-1/CD9) in breast carcinoma (BC, n = 19) and healthy breast (control, n = 5) tissues. Comparisons between the groups were drawn by the Mann-Whitney test; B, Pearson correlation between expression of MMP9 and CD9 in BC and healthy breast tissues.
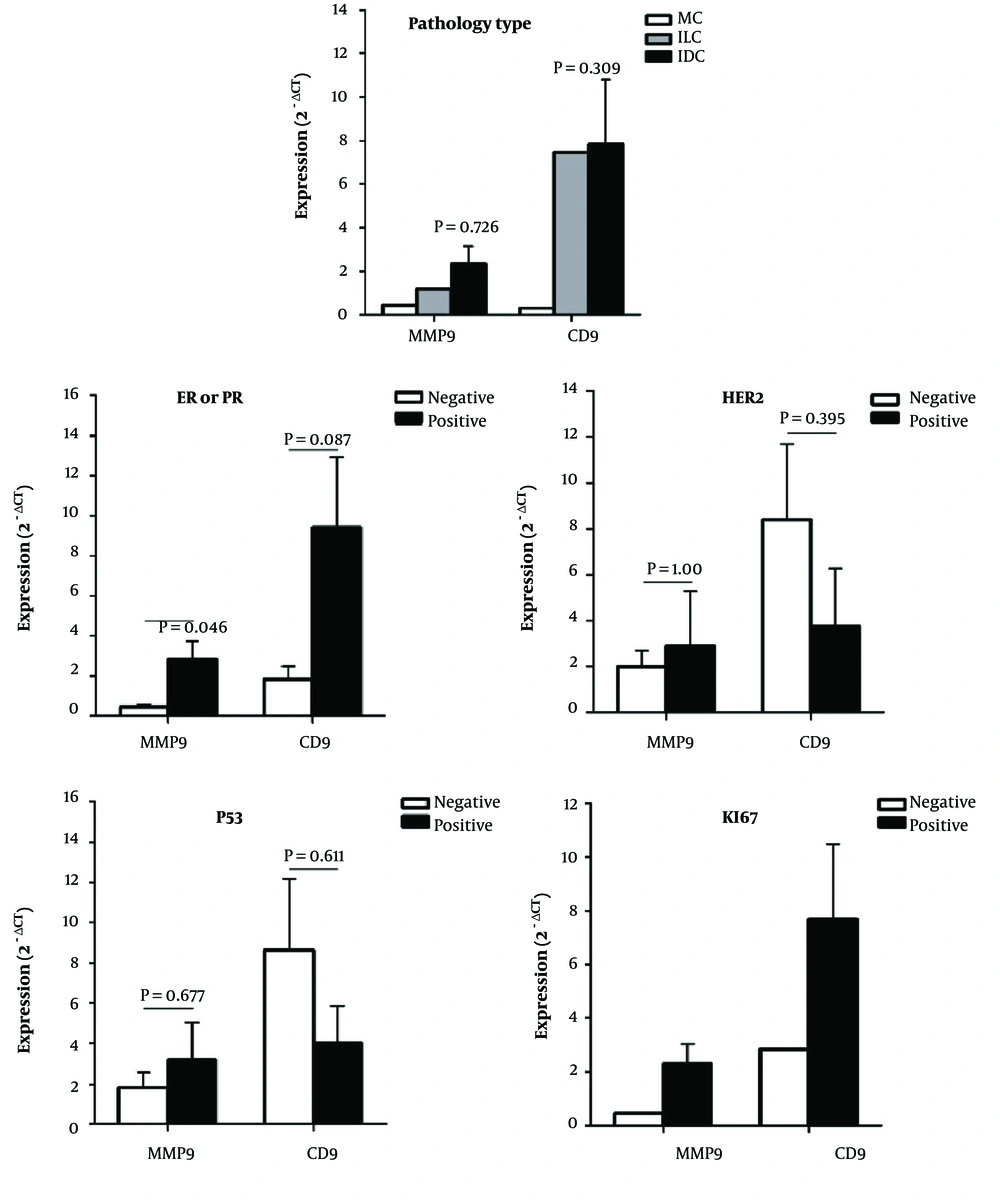
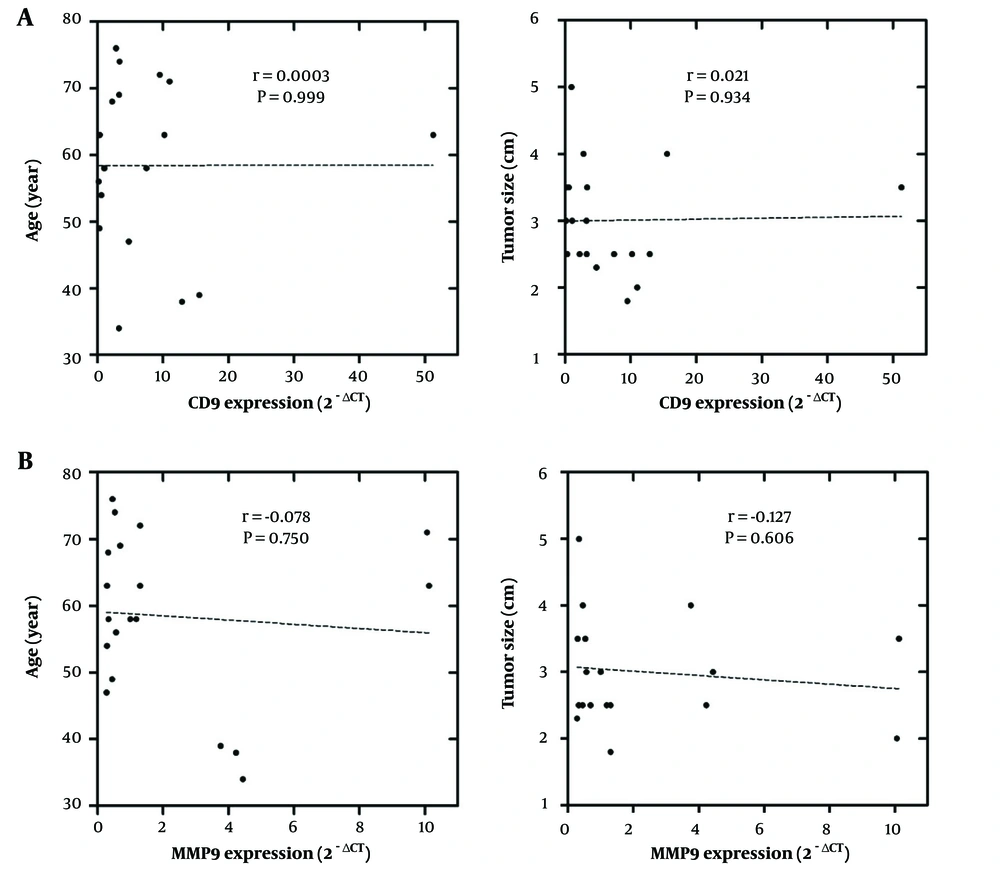
The association of MMP9 and CD9 expression with clinicopathological parameters including grade, pathology type, HER2, PR or ER, P53, and KI67 is presented in Figure 2. The MMP9 expression was significantly higher in ER or PR positive compared to ER or PR negative tumors (for both, P = 0.046). Although CD9 expression was higher in the ER or PR positive than in the ER and PR negative tumors, this difference was not statistically significant (for both, P = 0.087). Also, the CD9 expression was insignificantly lower in P53 positive than the P53 negative (P = 0.611) and HER2 positive than HER2 negative (P = 0.485) tumors. In addition, both MMP9 and CD9 showed overexpression in the grade II tumors compared to other grades (P = 0.311) and in KI67-positive compared to KI67-negative tumors; however, due to inadequate sample size, the statistical analysis was not feasible for KI67. Moreover, neither CD9 nor MMP9 expressions showed any correlation with patients’ age or tumor size (Figure 3).
5. Discussion
Over the past 2 decades, a growing body of evidence has shown that various mechanisms are involved in the progression of many cancers including BC. However, much remains to be done to uncover the mechanisms underlying the disease pathogenesis as well as the diagnostic value of involved molecules. Cell membrane proteins play a crucial role in the cells’ function, cell-to-cell, cell-matrix interactions, cellular signaling, and response to environmental factors, and have been implicated in many cancers. In the present study, we evaluated the expression status of MMP9 and CD9 in BC compared to normal breast tissues among the Iranian population. Our findings revealed no significant change in the expression of neither MM9 nor CD9 in BC compared to the normal breast tissue. However, a strong positive correlation was observed between the expressions of the two genes. Our findings contradict preceding studies that have reported down-regulation of CD9 and over-expression of MMP9 in human BC (23, 26).
Matrix metalloproteinase 9 is required for tumor growth, vascularization, and invasion in many cancers, and has been suggested as a potential diagnostic biomarker or therapeutic target for BC. Somiari et al. (25), for instance, observed the elevation of MMP9 in the serum of BC patients and suggested this molecule as a biomarker for disease classification. However, no change was observed in MMP9 expression in our study. Previous investigations of MMP9 in BC have revealed that this molecule is usually overexpressed in metastatic rather than non-metastatic disease. Daniele et al. (23) found higher expression of MMP9 in metastatic BC compared to non-metastatic and also normal tissues. Rahko et al. (26) showed that overexpression of MMP9 is an early marker of carcinogenesis preceding tumor invasion in human intraductal carcinomas of the breast. This might be a logical explanation for the unchanged MMP9 expression in our study, which did not include metastatic patients. Also, in agreement with this assumption, only MC patients showed very low MMP9 expression compared to ILC and IDC patients in our study. Additionally, Stankovic et al. (27) observed a significant increase in the expression and activity of the MMP9 enzyme in BC, while they did not observe any association between MMP-9 activity and HER2 expression, which is consistent with our finding. They also showed varying activity for MMP9 in different grades and stages of BC (27), which is in agreement with our study, as we also found higher MMP9 expression in grade II tumors compared to grades I and III. Moreover, Mehner et al. (28) have reported higher MMP9 expression in basal-like triple-negative (HER2- ER- PR-) breast tumors suggesting a driver for a malignant phenotype. Furthermore, Li et al. (29) and Sullu et al. (30) have found over-expression of MMP9 in high-grade, ER-negative, or triple-negative tumors suggesting it as a strong diagnostic biomarker for BC. In contrast, we herein observed significant overexpression of MMP9 in either ER+ or PR+ BC patients compared to the negative counterparts.
The cell-surface protein MRP-1/CD9 acts in many cellular processes, including differentiation, adhesion, and signal transduction. It was initially reported as a suppressor of metastasis so that any reduction in its expression had been implicated in many human cancers including BC (13-17). Arihiro et al. (31) found loss of CD9 expression to be associated with lymph node metastasis in BC. On the other hand, recently, overexpression of CD9 has also been reported to be associated with an increased risk of bone metastasis in BC, suggesting a role for this molecule in homing the metastatic cancer cells (19). More recently, Rappa et al. (20) demonstrated that CD9 knockdown reduced the formation of metastasis in animal models of BC. In the present study, we did not observe any significant alterations in the CD9 expression in BC tumors. But we found decreased CD9 expression in HER2-positive compared to HER2-negative tumors, while increased CD9 expression in PR or ER-positive tumors. In agreement with previous studies, our observations indicate that CD9, like other tetraspanins, might selectively act as either a tumor suppressor or promoter in different tumor types or certain steps of BC progression and metastasis (19, 32, 33). What’s more, we found a strong direct correlation between CD9 and MMP9 expression in BC tumors. This casts doubt on the tumor suppressor role of CD9 and further support its metastasis-promoting activity (19, 33).
To summarize, we found no significant changes in the expression of MMP9 and CD9 in BC tissues compared to the normal breast tissues. However, we observed varying expressions of MMP9 and CD9 depending on the tumor clinicopathological features, which cast doubts on the potential of these molecules to be accurate biomarkers or therapeutic targets for BC. Importantly, we found a strong correlation between expressions of CD9 and MMP9 in BC tissues, which seemingly is in favor of the tumor-promoting activity of CD9 versus its tumor suppressor function. However, much more studies are required to further support this conclusion.