1. Background
Breast cancer is one of the most frequent cancers (1) and the main cause of cancer-related deaths (14%) among women all over the world (2-4). In addition to environmental agents, body weight (2) and lifestyle factors, such as alcohol consumption, physical inactivity, smoking, (5) and genetic background may have an important effect in developing breast tumors (5, 6). Researchers have demonstrated that, in addition to unlimited cell proliferation, the suppression of apoptosis correlated with the development of various tumors (3). Apoptosis, significantly, regulates various critical cellular and biological processes, such as organ development, homeostasis, and tumor cells destruction (7, 8). Abnormal regulation of apoptotic pathways and consequent deregulation of mentioned biological processes lead to the development of human malignancies (3). Death receptor on the surface of many cell types, fas and its ligand, fasL as members of the tumor necrosis factor superfamily (TNF), interact to initiate the extrinsic death signal pathway, which consequently leads to apoptotic cell death (7, 9-12). Structural alterations of fas and fasL may influence the expression of them and consequently result in the development of various tumors, such as breast tumors (7, 13). The human fas on 10q24.1 (12, 14), which consists of 9 exons and 8 introns, (14) has 2 single nucleotide polymorphisms (SNPs) in its promoter. One of them is a G-A transition at nucleotide number -1377 (fas-1377G > A) in the silencer region, and the other is an A-G transition at nucleotide number -670 (fas-670A > G) in the enhancer site. By electrophoretic mobility shift assay (EMSAs), the previous studies reported that reduced binding of Sp1 and STAT1 has been observed, respectively, in the presence of fas-1377A and fas-670G alleles, which may decrease transcription and translation of the fas (2, 15). Some studies showed that fas promoter SNPs correlated with various malignancies, such as breast cancer (2, 16). Few surveys investigated the correlation between fas promoter polymorphisms and breast cancer risk. Hence, the mentioned association is almost unknown. To investigate the effect of fas promoter polymorphisms on the risk of breast cancer, we genotyped the fas-1377G > A, fas-670A > G substitutions and evaluated their association with breast cancer risk in northwest of Iran. Moreover, in the present study, in silico application was used in order to detect the alterations of protein binding pattern in the fas sequence around SNPs.
2. Methods
2.1. Patients with Cancer and Healthy Controls
This case-control study was performed on 200 women with breast cancer and 186 healthy women without cancer history among their relatives. All of the patients had undergone mastectomy/lumpectomy at Imam Reza and Noor-E-Nejat Hospitals in Tabriz, Iran between 2008 and 2012. All participants were from northwest provinces of Iran. In the current study, northwest of Iran includes 4 provinces, including East Azarbaijan, West Azarbaijan, Ardabil, and Kurdistan, according to the official map of Iran. The extraction of DNA was performed on all subjects’ peripheral blood samples, using SDS/proteinase K and salting-out method (17). We did not use DNA extraction kit in this study. Blood drawing and DNA extraction were performed with consent from all subjects as well as permit number 5.4.3259/13.3.92 (2013) from the 13th ethics committee of Tabriz University of Medical Sciences research center, which were concordant with the Helsinki declaration. The clinicopathological characteristics were obtained from medical records. Tumor-Node-Metastasis (TNM) staging was applied for tumor staging (18).
2.2. Primers Selection and PCR Amplification
Genotyping of fas-1377G > A and fas-670A > G was done, using tetra-amplification refractory mutation system-polymerase chain reaction (Tetra-ARMS-PCR) and restriction fragment length polymorphism-polymerase chain reaction (RFLP-PCR) (19), respectively.
2.3. Tetra-ARMS-PCR
Sequences of the primers were designed according to the previous studies (2); amplicon sizes are shown in Table 1. The amplification of both wild-type and polymorphic alleles was, simultaneously, conducted in a single-tube PCR. In order to detect fas-1377G > A, PCR amplification was prepared in a total volume of 25 µL containing: 1 µL template DNA (average concentration of 200 ng), 0.8 µL of each primer (10 pM), 2.5 µL of reaction buffer 10x, 0.5 µL of deoxyribonucleotide triphosphate (dNTP), 0.85 µL of Mgcl2, and 0.2 µL of Taq DNA polymerase (Cinnagenn, Tehran, Iran). PCR program was run at 95°C for 5 minutes, followed by 30 cycles of a denaturation for 30 seconds at 95°C, annealing for 30 seconds at 64°C, extension for 30 seconds at 72°C, and a final extension for 10 minutes at 72°C in a thermo-cycler (Sensoquest, GmbH, Germany). Amplicon sizes for G and A alleles were 216 bp and 340 bp, respectively. The products were analyzed by gel electrophoresis on a 2% agarose gel containing ethidium bromide.
| Primers | Sequence (5’ → 3’) | dbSNP |
|---|---|---|
| Forward outer primer (FO) | 5’-CCTTCCCTCACACCCCTTTTCCTTCC-3’ | rs2234767 |
| Reverse outer primer (RO) | 5’-CTTTGGCATCGTCCACCAAGCTCTG-3’ | |
| Forward inner primer (FI), (A allele) | 5’-AGTGTGTGCACAAGGCTGGCCCA-3’ | |
| Reverse inner primer (RI), (G allele) | 5’-TTAGTGCCATGAGGAAGACCCTGTGC-3’ | |
| Forward primer (F) | 5’-ATAGCTGGGGCTATGCGATT-3’ | rs1800682 |
| Reverse primer (R) | 5’-CATTTGACTGGGCTGTCCAT-3’ |
2.4. RFLP-PCR
The fas-670A > G genotypes were determined, using RFLP-PCR. Sequences of the primers, as described earlier, (20) are shown in Table 1. Primers for this SNP produce a 193 bp fragment. Amplification was done under the following conditions: A 25 µL reaction mixture contained 2.5 µL of reaction buffer 10x, 1 µL of DNA, 0.9 µL of each primer (10 pM), 0.5 µL of dNTP, 0.76 µL of Mgcl2, and 0.18 µL of Taq. The enzyme, Bme1390I (ScrFI) (Fermentas, USA), was used to distinguish the fas-670A > G. ScrFI digestion generated the following fragments: fas-670A allele, a single fragment of 193 bp; and fas-670G, fragments of 136 bp, and 57 bp (gain of ScrFI digestion site).
2.5. In Silico Analysis
PNI modeler, as an online web program (available on http://165.246.44.34/PNI modeler/), predicts nucleotides that bind to proteins. This application was used to indicate whether the fas-1377G/A and fas-670A/G influence the protein binding sites in the promoter of fas.
2.6. Statistical Analysis
Genotypes and alleles distributions were compared between patients and controls by Pearson’s Chi-square or Fisher’s exact tests. The strength of the association between the polymorphisms and breast cancer was obtained by odds ratios (ORs) with 95% confidence intervals (95% CIs). The java stat online statistics package (available on http://statpages.org/ctab2x2.html) was used to calculate mentioned statistical tests. Haplotype analysis was performed, using SHEsis software (available on http://analysis.bio-x.cn/myAnalysis.php). The SHEsis software was also used to calculate the Linkage disequilibrium (D’) and correlation coefficient (r2) between 2 polymorphic sites and check Hardy-Weinberg equilibrium (HWE) in controls based on Pearson’s Chi-square test. For all examinations, P value < 0.05 was a significant result. The mean values were calculated by statistical package for the social sciences (SPSS) software (v.16). Based on Bonferroni correction test, adjusted P value reported would be 0.005.
3. Results
3.1. Subjects’ Characteristics
The average standard deviation age was 47.93 ± 10.03 for cases and 42.49 ± 12.51 for controls; all participants were women. Clinical characteristics of the patients are indicated in Table 2. According to clinicopathological information, 9 patients had ductal carcinoma in situ (DCIS), 181 of them had invasive ductal carcinoma (IDC), and 10 patients had invasive lobular carcinoma (ILC).
| Characteristics | Cases | Controls |
|---|---|---|
| Mean age ± standard deviation | 47.93 ± 1 0.03 | 42.49 ± 12.51 |
| Range of age (year) | 25 - 81 | 19 - 79 |
| Tumor-type | ||
| DCIS | 9 (4.5) | - |
| IDC | 181 (90.5) | - |
| ILC | 10 (5) | - |
| Tumor-stage | ||
| Stage 0 | 13 (6.5) | - |
| Early (I and II) | 85 (42.5) | - |
| Late (III and IV) | 94 (47) | - |
| Unknown | 8 | |
| Tumor grade | ||
| I | 38 (19) | - |
| II | 122 (61) | - |
| III | 20 (10) | - |
| Unknown | 20 | |
| Lymph-node metastases | ||
| Positive | 114 (57) | - |
| Negative | 76 (38) | - |
| Unknown | 10 | |
| Tumor-size | ||
| ≤ 3.9 | 98 (49) | - |
| > 3.9 | 91 (45.5) | - |
| Unknown | 11 | |
| Side-involved | ||
| Right | 95 (47.5) | - |
| Left | 94 (47) | - |
| Both | 11 (5.5) | - |
aValues are expressed as No. (%).
3.2. Association of Fas Polymorphisms and Breast Cancer
The allele/genotype distributions of fas-1377G/A,-670A/G in patients and controls are shown in Table 3. For fas-1377G > A, the GG genotype had a higher frequency in controls rather than patients (65.6% and 60%, respectively). In contrast, the frequency of GA genotype in patients was 7% higher than controls (OR = 1.338; 95% CI = 0.854 - 2.097; P = 0.181). For fas-670A > G, homozygous genotype for A allele showed 7.47% higher frequency in controls than cases, while heterozygous genotype had more frequency among patients (OR = 1.408; 95% CI = 0.895 - 2.217; P = 0.118). The distribution of the fas-1377GG, GA, AA, and fas-670AA, AG, GG genotypes among patients was not significantly different from those among the controls (P > 0.05). Genotype distribution of fas-1377A > G/-670G > A in controls was in consistence with Hardy-Weinberg equation (HWE) (P > 0.05). Thus, observed genotype distribution in healthy subjects represented the genotype frequency in the overall northwest population of Iran. Allelic frequencies were also calculated for each polymorphism. The allele frequencies for fas-1377A and fas-670G were 19.09% and 31.72% in controls compared with 21.25% and 35.5%, in patients, the frequencies for fas-1377G and fas-670A were 80.91% and 68.28% in controls compared with 78.75% and 64.5% in patients, respectively. The observed differences for frequency of alleles between cases and controls were not significant (P > 0.05). Eight combined genotypes of fas were obtained in patients and healthy subjects (Table 4). The most frequent combined genotype in both groups was fas-1377GG/-670AA (∆ = 7.7%). Because 2 polymorphisms in fas (-1377G > A/-670A > G) were in linkage disequilibrium with each other (D’ = 0.798, r2 = 0.318), the combined association of them with breast cancer risk was calculated by haplotype analysis. The frequency of fas (-1377/-670) haplotypes did not show a significant difference between cases and controls (P > 0.05) (Table 4).
| Polymorphisms | Breast Cancer: No. (%) | Control: No. (%) | OR (95%CI) | P Valuea |
|---|---|---|---|---|
| fas-1377A > G | ||||
| GG | 120 (60) | 122 (65.6) | Ref | - |
| GA | 75 (37.5) | 57 (30.64) | 1.338 (0.854 - 2.097) | 0.181 |
| AA | 5 (2.5) | 7 (3.76) | 0.726 (0.194 - 2.638) | 0.592 |
| GA + AA | 80 (40) | 64 (34.40) | 1.271 (0.822 - 1.965) | 0.256 |
| Alleles | ||||
| G | 315 (78.75) | 301 (80.91) | Ref | - |
| A | 85 (21.25) | 71 (19.09) | 1.144 (0.792 - 1.653) | 0.454 |
| fas-670G>A | ||||
| AA | 84 (42) | 92 (49.47) | Ref | - |
| AG | 90 (45) | 70 (37.63) | 1.408 (0.895 - 2.217) | 0.118 |
| GG | 26 (13) | 24 (12.9) | 1.187 (0.604 - 2.332) | 0.594 |
| AG + GG | 116 (58) | 94 (50.5) | 1.352 (0.886 - 2.062) | 0.141 |
| Alleles | ||||
| A | 258 (64.5) | 254 (68.28) | Ref | - |
| G | 142 (35.5) | 118 (31.72) | 1.185 (0.868 - 1.616) | 0.267 |
Abbreviations: CI, Confidence Interval; OR, Odds Ratio.
ax2-test.
| fas-1377G > A | fas-670A > G | Cases No. (%) (N = 200) | Controls, No. (%), (N = 186) | OR (95% CI) | P Value |
|---|---|---|---|---|---|
| G/G | A/A | 76 (38) | 85 (45.7) | Ref | - |
| G/G | A/G | 27 (13.5) | 28 (15.06) | 1.078 (0.559 - 2.081) | 0.809 |
| G/G | G/G | 17 (8.5) | 9 (4.84) | 2.113 (0.828 - 5.487) | 0.085 |
| G/A | A/A | 8 (4) | 7 (3.76) | 1.278 (0.397 - 4.147) | 0.649 |
| G/A | A/G | 61 (30.5) | 42 (22.58) | 1.505 (0.763 - 2.977) | 0.205 |
| G/A | G/G | 6 (3) | 8 (4.30) | 0.839 (0.245 - 2.821) | 0.755 |
| A/A | A/G | 2 (1) | 0 | - | 0.227 |
| A/A | G/G | 3 (1.5) | 7 (3.76) | 0.479 (0.094 - 2.158) | 0.344 |
| Pairwise haplotypes | Cases | Controls | OR (95%CI) | P value | |
| A-1377 | A-670 | 0.032 | 0.022 | 1.490 (0.615 - 3.614) | 0.37 |
| A-1377 | G-670 | 0.180 | 0.169 | 1.081 (0.745 - 1.568) | 0.68 |
| G-1377 | A-670 | 0.613 | 0.661 | 0.812 (0.605 - 1.089) | 0.16 |
| G-1377 | G-670 | 0.175 | 0.148 | 1.217 (0.828 - 1.789) | 0.31 |
| D’ (r2), 0.763 (0.285) | 0.831 (0.351) | ||||
3.3. In Silico Analysis
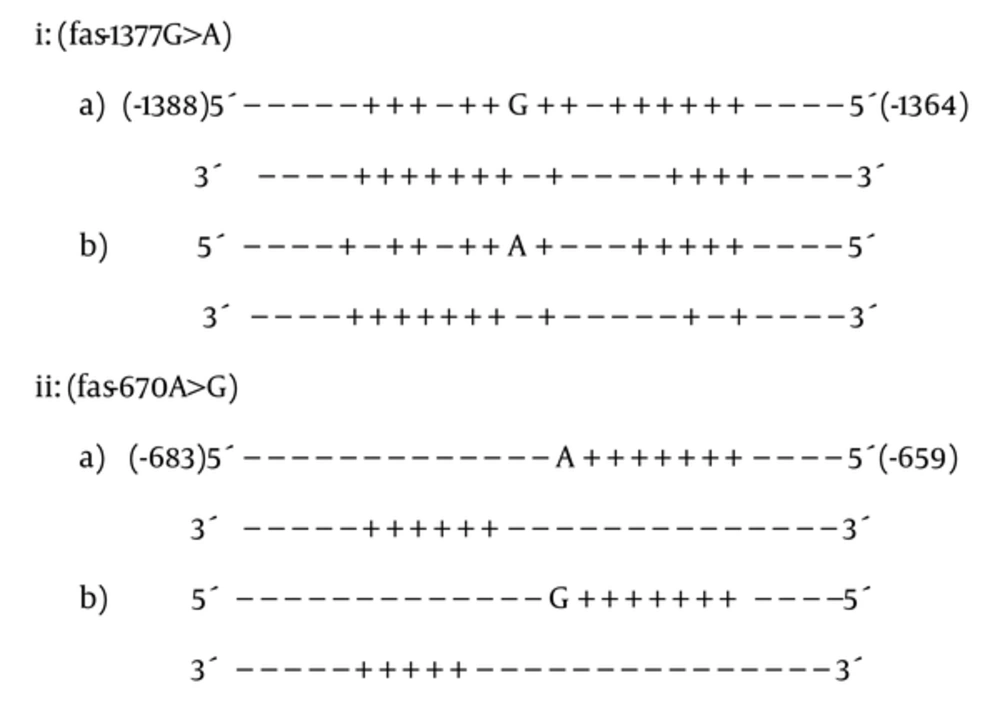
Based on PNI modeler results (Figure 1, i, ii), the fas-1377G > A and fas-670A > G altered protein interaction pattern of the promoter sequence around both polymorphisms that might also alter transcription factor binding to the promoter of fas and might change gene transcription and translation. For fas-1377G > A, PNI modeler revealed that there was 1 shift to the left for probable protein interaction site in upstream region of nucleotide -1377G (i). Moreover, there were 2 lost protein interaction sites around nucleotide -1377G. For -1377G > A and -670A > G, 2 (i) and 1 (ii) interaction sites vanished respectively, that both of them were seen in complementary DNA sequence.
PNI Modeler Prediction Results, - and + signs determine non-interaction and protein interaction nucleotides, respectively. Polymorph alleles are shown in bold face. Fas DNA duplex that have G nucleotide in site -1377 (i a); fas DNA duplex with exchanged nucleotide A in site -1377 (i b); fas DNA duplex that have A nucleotide in site -670 (ii a); fas DNA duplex with exchanged nucleotide G in site -670 (ii b).
4. Discussion
Death receptor and its ligand interact to initiate the extrinsic apoptotic pathway that in some cell types links to the intrinsic pathway of apoptosis through Bid protein (20). The fas is expressed on the tumor cells that can be assistant to the Fas-triggered destruction of tumor cells by the immune system. Some studies showed that the fas expression was decreased on breast tumor cells; this event may be due to the fas polymorphisms (2, 21). Structural changes of fas, including 2 common polymorphisms of its promoter (2) were seen in various human malignancies, such as T-cell leukemia, (22) urinary bladder, (23) non-small cell lung cancer (24), and malignant melanoma, (25) as well as breast cancer (2) and autoimmune diseases, such as rheumatoid arthritis (26). To the best of the authors’ knowledge, as the first case-control study in northwest of Iran, we investigated the correlation between 2 common polymorphisms in the promoter of fas. The present study did not show any significant association between the risk for breast cancer and fas-670A > G. The results of studies on breast cancer in Chinese population (3) and prostate cancer in Portuguese population (27) revealed that the fas-670GG genotype had a protective effect on the development of breast and prostate cancers. Recently, Xu et al. applied a meta-analysis of cancer cases and controls, including fas-1377G > A and fas-670A > G from 52 case-control studies, with similar results to the present study; the results showed no significant association between fas-670A > G and breast cancer risk that might have variability based on various tumor types, examined population, and different technics in genotyping assay. Therefore, this explanation may be a reason for different observed results, even conflicting, among various investigations (28). Several studies have analyzed the correlation between fas-1377G > A and human cancers. Studies on childhood acute lymphoblastic leukemia (15) and hepatitis B virus infection (29) in Iran have shown no significant association in fas-1377G > A and fas-670A > G with the risk of both diseases that were in agreement with the findings of this study. Some of the studies have demonstrated that the A allele has a higher frequency among cases than healthy subjects, (5, 12); however, contrastingly in some others, the A allele had a higher frequency in controls in comparison with patients (8, 16, 30). In the present study, the frequency of fas-1377A allele and GA genotype was higher in patients rather than the control group, but the differences were not significant. Several meta-analyses have examined the relationship between fas-1377G > A and breast cancer risk. Some of them have demonstrated that the fas-1377G > A polymorphism is associated with higher sensitivity for breast cancer (28, 31, 32). Turkish group’s investigation revealed that homozygosity for fas-1377G was associated with higher risk of bladder cancer (33). Crew et al. reported that the fas-1377G > A showed no different frequency between breast cancer patients and controls in Long Island, New York (16). Hashemi et al. also observed the same result in south of Iran (2). The results of the present study were in agreement with the results of the survey conducted by Crew et al. (16) and Hashemi et al. (2). Several investigations have shown the correlation of fas promoter haplotypes with the cancer development risk among various ethnic groups. Zhang et al. reported a significant more frequency for (-1377A-670A) haplotype among patients rather than controls, whereas their study showed a higher frequency for (-1377G-670G) haplotype among controls in comparison with patients (5). In the present study, the -1377G-670A had a higher frequency in controls rather than in patients, but the difference was not significant. In the United Kingdom, and in patients with acute myeloid leukemia, this was the same about -1377G-670A, and there was a significant higher frequency among patients in comparison with controls for -1377A-670A (15). In Chinese patients with breast cancer, fas-1377A/-670A had a significant higher frequency among controls in comparison with patients; however, the same study showed an increased risk of breast cancer in subjects with the fas-1377G/-670A (3). In addition to SNPs analysis, we investigated the correlation between risk of breast cancer and combined genotypes in the fas promoter. Results of the present study showed that the -1377GG-670AA had, noticeably, a higher frequency in controls rather than patients. The contrast result was obtained about -1377GA-670AG that was more frequent among patients, but the differences were not significant for both of combinations. To the best of the authors’ knowledge, none of the previous studies have demonstrated the association between genotype combination status of 2 most common fas promoter polymorphisms and cancer risk to make a comparative interpretation (2, 3, 5, 9). In addition, the genotype frequency in Iranian control subjects was consistent with the results on other diseases in Iran. For example, the frequencies of the fas-1377GG,GA, and AA in our control group were 65.59%, 30.64%, and 3.76%, respectively, which was similar to those found by Mohammadi et al. 72%, 25%, and 3%, respectively. The frequency of the fas-1377A allele in our healthy subjects was 19.09%, which was also similar to that reported by Mohammadi et al. (29), 15.5%. The agreement between the data of this study and those of other studies with Iranian subjects suggests that any genotyping bias in the estimation of the variant allele frequencies is not substantial (29). Polymorphisms within promoter may influence transcription factor interaction with DNA sequence (34). The position of -1377G > A/-670A > G is in the promoter of fas that may influence protein binding to DNA. It was shown that the reduced fas expression level on cells is often correlated with various malignancies, such as breast tumors (5). It was believed that the transcription factors had an important role in balancing transcription and translation genes. It was reported that transcription factors, Sp1, STAT1, (5) ADD1/SREBP1 (35), and P53 (36) are correlated with the fas transactivation. Recently, in silico applications were used to analyze the polymorphisms effects in gene functions (37). In the present study, PNI modeler results showed that the fas promoter transitions (-1377G > A/-670A > G) altered protein binding pattern in the promoter around SNPs that might also change the interaction of transcription factors, such as Sp1/STAT1 with the fas promoter and might result in postulated altered fas transcription. To the best of the authors’ knowledge, none of the previous studies have investigated the effect of fas promoter polymorphisms on the binding pattern of proteins through in silico analysis. The present investigation has some limitations. One of the limitations of this study is the lack of environmental agents that effect determination, such as diet, physical activity, the use of oral contraceptives, which may influence breast cancer risk via gene-environment interactions. Insufficient patient population for further sub-group studies and the lack of family history information of patients could be another limitation of this study. One of the other limitations of this study is the lack of investigation about the effect of -1377G > A-670A > G haplotypes on cancer initiation/progression in molecular level. If the results of this study are consistent with the results of large studies and supported by in vitro investigations, they can be useful in the determination of relative risk of breast tumors development in northwest of Iran.
4.1. Conclusions
In conclusion, the results of this study did not show any significant associations between 2 polymorphisms of fas promoter and breast cancer risk. However, further investigations are required to validate these conclusions across different populations. Moreover, PNI modeler program showed that the binding pattern of proteins to the promoter of the fas gene changes due to the presence of -1377G > A, -670A > G polymorphisms in the promoter.