1. Background
During the recent 2 decades, the rate of ductal carcinoma in situ (DCIS) has severely increased following the extensive implementation of screening mammography. One out of 3 new breast cancer cases is diagnosed annually (1). According to the local regional treatment pattern of DCIS in a recent surveillance, epidemiology, and end results (SEER) analysis, 69.5% and 43% of the patients were treated by breast conserving surgery (BCS) and radiotherapy following BCS or lumpectomy alone, respectively (2). Four prospective randomized III trials have shown the effectiveness of radiotherapy in reducing breast recurrence following lumpectomy (3-6).
Many retrospective analyses suggested outstanding findings with hypofractionated whole breast radiation, as well as partial breast irradiation in women suffering from DCIS (7-9). The majority of the local recurrences following BCS and radiotherapy for DCIS appear in the neighborhood of the main tumor and about half of them are invasive.
Investigating RTOG 9804, McCormick et al. (10) evaluated the effect of radiotherapy in good-risk patients, in which it closed sooner because of its brief results in the role of radiotherapy even in good-risk patients (with the mean follow-up of 7.17 years and 6.7% and 0.9% of recurrence rate in the surgery alone group in comparison with the surgery plus radiotherapy group, respectively).
Rashtion et al. (11) treated 23 patients with DCIS with intraoperative radiotherapy (IORT) after lumpectomy. There was only 1 local recurrence in the 3-year follow-up. The patient had a high-grade DCIS and did not use hormone therapy. They concluded that, in special conditions, IORT can be an alternative for whole breast radiotherapy (WBRT) in patients with DCIS (11). In another study that investigated patients with DCIS in our center, 40 patients received EBRT and 29 patients received IORT. Local recurrence was significantly greater in the IORT group (17.25 vs 5.4%) because the intraoperative electron radiotherapy (IOeRT) group contained biologically high-risk patients and the patient selection criteria in the IRIORT consensus was not observed. Overall, 59.3% and 58.6% of the patients in the IOeRT group had high-grade and hormone receptor-negative tumors, respectively. Five local recurrences were reported; 2 cases had 3-cm and 4.5-cm tumors and 1 case was younger than 40 years old (12). In Stephanie et al.’s study carried out in North America, IORT was considered for 935 patients with breast cancer, 9% (95 patients) of whom were DCIS and 26% (25 patients) received supplemental external beam radiotherapy (EBRT). Local recurrence occurred in 3% (3 patients) with a mean follow-up of 1.5 years (13).
2. Objectives
In this study, we delivered IORT with 2 types of electron beams and photon, which were delivered by LIAC (made in Italy) and INTRABEAM ZEISS (invented in America) mobile linear accelerators, respectively. We divided patients into radical and boost subgroups according to the IRIORT consensus criteria (Table 1). We attempted to evaluate the results of IORT for patients with DCIS compared with those who received EBRT.
3. Methods
We studied 138 breast cancer patients with DCIS histology treated in 3 centers affiliated to the Cancer Research Center of Shahid Beheshti University from September 2013 to September 2019. The study was approved by the Ethics Committee of the Cancer Research Center, Shahid Beheshti University of Medical Sciences. Written informed consent was taken from the patients.
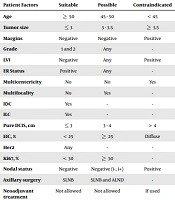
Patients who received IORT were divided into two subgroups based on their clinical, pathological, and biological characteristics to receive radical or boost dose of radiotherapy by IORT according to the IRIORT consensus criteria (Table 1); they, then, were compared with the EBRT group.
| Patient Factors | Suitable | Possible | Contraindicated |
|---|---|---|---|
| Age | ≥ 50 | 45 - 50 | < 45 |
| Tumor size | ≤ 3 | 3 - 3.5 | ≥ 3.5 |
| Margins | Negative | Negative | Positive |
| Grade | 1 and 2 | Any | - |
| LVI | Negative | Any | Positive |
| ER Status | Positive | Any | - |
| Multicentericity | No | No | Yes |
| Multifocality | No | Yes | - |
| IDC | Yes | - | - |
| ILC | Yes | - | - |
| Pure DCIS, cm | ≤ 3 | 3 - 4 | > 4 |
| EIC, % | < 25 | ≥ 25 | Diffuse |
| Her2 | Any | - | - |
| Ki67, % | < 30 | ≥ 30 | - |
| Nodal status | Negative | Negative (i-, i+) | Positive |
| Axillary surgery | SLNB | SLNB and ALND | - |
| Neoadjuvant treatment | Not allowed | Not allowed | If used |
Abbreviations; ALND, axillary lymph node dissection: EIC, extensive in situ component: LVI, lymphovascular invasion: SLNB, sentinel lymph node biopsy.
The EBRT group included 57 patients, who received 45 - 50 Gy in 25 fractions of EBRT within 5 to 6 weeks and, then, 10 Gy in 5 fractions of boost dose 4 weeks after BCS. The radical group included 45 patients; 36 and 9 patients of this group received IOeRT and intraoperative X-ray radiotherapy (IOxRT) as radical dose, respectively. There existed 36 patients in the boost group; 15 and 21 patients of this group received IOeRT and IOxRT as boost dose, respectively.
For delivering IOeRT, if patients had suitable and possible criteria according to the IRIORT consensus criteria, they would receive 21 Gy electron as radical dose, otherwise, they would receive 12 Gy as boost dose, followed by supplemental EBRT.
20 Gy 50 kV IOxRT was delivered after the report of clear margins in the frozen section. After the final pathology report, if patients had demographic, pathologic, and biological criteria of the suitable and possible group in IRIORT, they would be considered as radical dose, otherwise, they would be considered as boost dose and the patients would receive supplemental EBRT. The patients, who could not complete the treatment process or forgo it, were excluded from the study.
The patients were visited by the surgeon and radio oncologist every 6 months until 2 years and yearly thereafter; then, they underwent mammography 1 year after surgery and every 2 years thereafter. If they were not visited by the surgeon and radio oncologist in the recent year, we would ask their last condition by telephone call.
Disease-free survival (DFS) is described as diagnosing time until recurrence (local and distant) time and overall survival (OS) is described as diagnosis time until the last follow-up or death.
The present longitudinal non-randomized cohort study evaluated the distant metastasis and local recurrence in patients with DCIS with the mean follow-up of 37 and 40.1 months for the IORT and EBRT groups, respectively. The current research was carried out from September 2013 to September 2019 in our center. The primary endpoints were recurrence (local and distant) and death and the secondary endpoints were the role of age, tumor size, grade, and hormone receptor (HR) factors in recurrence and death by univariate and multivariate Cox proportional hazards regression model.
The survival plots and the cumulative hazard function were drawn, using the Kaplan-Meier method. The log-rank test was used to evaluate the survival difference between the two treatment radiotherapy groups, as well. The data were analyzed by SPSS.
4. Results
Out of 138 patients studied with DCIS, 45 patients received radical dose of IORT (36 patients received IOeRT and 9 patients received IOxRT), 36 patients received boost dose of IORT (15 patients received IOeRT and 21 patients received IOxRT), and 57 patients received EBRT.
Table 2 presents the characteristics of patients and tumors of the EBRT and IORT groups.
| Groups | |||
|---|---|---|---|
| EBRT, 57 (100) | Boost, 36 (100) | Radical, 45 (100) | |
| Size, cm | |||
| ≤ 2.5 | 42 (91.30) | 19 (52.80) | 35 (85.4) |
| 2.5 - 3 | 4 (8.70) | 4 (11.10) | 3 (7.30) |
| > 3 | 0 (0.00) | 13 (36.10) | 3 (7.30) |
| Total | 46 (100.00) | 36 (100.00) | 41 (100.00) |
| Grade | |||
| 1 | 12 (54.50) | 4 (14.80) | 5 (20.80) |
| 2 | 8 (36.40) | 9 (33.30) | 15 (50.00) |
| 3 | 2 (9.10) | 14 (51.90) | 7 (29.20) |
| Total | 22 (100.00) | 27 (100.00) | 27 (100.00) |
| Estrogen receptor | |||
| Positive | 23 (65.70) | 20 (57.10) | 30 (76.90) |
| Negative | 12 (34.30) | 15 (42.90) | 9 (23.10) |
| Total | 35 (100.00) | 35 (100.00) | 39 (100.00) |
| Progesterone receptor | |||
| Positive | 20 (58.80) | 9 (64.30) | 20 (69.00) |
| Negative | 14 (41.20) | 5 (35.70) | 9 (31.00) |
| Total | 34 (100.00) | 14 (100.00) | 29 (100.00) |
| Age | |||
| < 40 | 6 (10.70) | 10 (27.80) | 2 (4.40) |
| 40 - 50 | 21 (37.50) | 12 (33.30) | 10 (22.20) |
| ≥ 50 | 29 (51.80) | 14 (38.90) | 33 (73.30) |
| Total | 56 (100.00) | 36 (100.00) | 45 (100.00) |
aValues are expressed as No. (%).
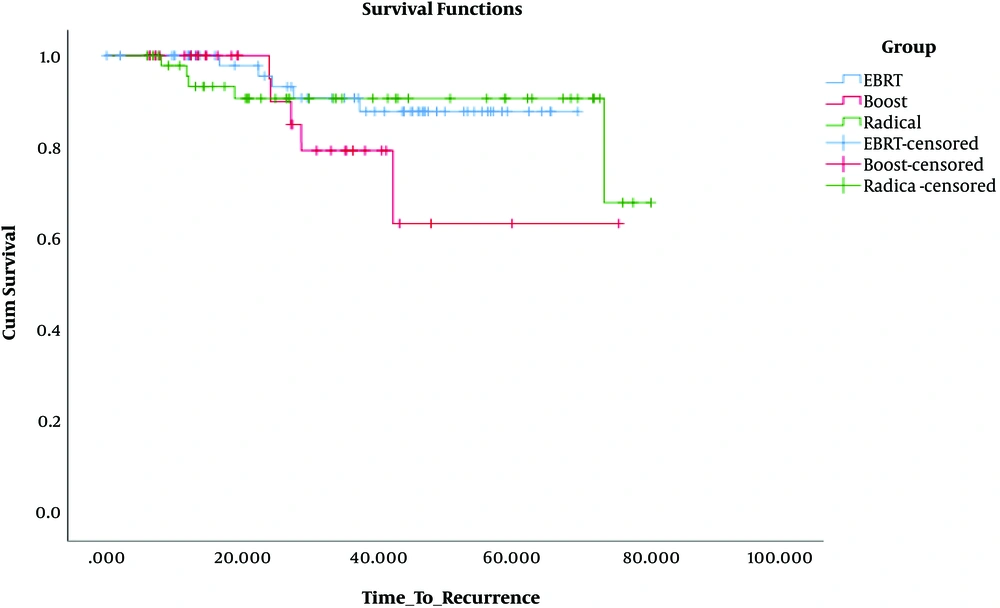
Local recurrence occurred in 8.8% (5 patients), 13.9% (5 patients), and 2.2% (1 patient) of the patients of the EBRT, boost, and radical groups, respectively. Of 5 patients in the EBRT group, who had a recurrence, 3 had hormone receptor-negative tumors, 3 had grade 1 tumor, and 2 were aged 40 to 50 years. Of 5 patients in the boost group that showed recurrence, 3 were younger than 40 years, 2 had hormone receptor-negative tumors, 2 had a high-grade tumor, and 2 had tumors with 6 cm size. The patient in the radical group that showed recurrence, had high grade and hormone receptor-negative tumor. There was no metastasis or death in the groups.
DFS was 87.8%, 63.5%, and 100% in the EBRT, boost, and radical groups, respectively.
The log-rank test was used to assess the DFS difference between the treatment groups. No significant difference was observed in the boost (P = 0.12) and radical (P = 0.95) groups compared with the EBRT group. But, when we compared radical and boost groups, the radical group had a better DFS (P = 0.007) (Figure 1).
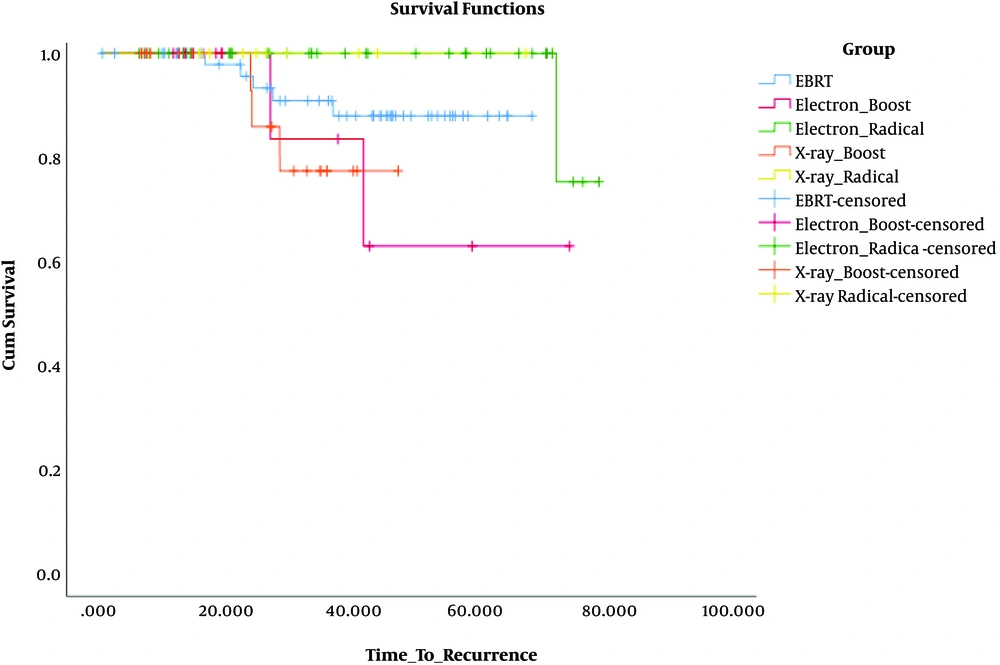
Not only was there no significant difference in DFS when we compared two types of beams (electron and photon) as radical and boost dose with EBRT [in comparison of electron radical with EBRT (P = 0.08), X-ray radical with EBRT (P = 0.4), electron boost with EBRT (P = 0.23), and X-ray boost with EBRT (P = 0.26)], but also no significant difference in DFS was observed in comparison of beams as radical and boost dose with each other [in comparison of electron boost with X-ray boost (P = 0.93) and because of no recurrence in X-ray radical group, its comparison with electron radical was not calculable].
When we compared beams as radical and boost dose, electron beam as radical dose had a better DFS than as boost dose (P = 0.045), but it was not for X-ray beam (P = 0.25).
The most difference in DFS was observed when we compared the electron radical group with the X-ray boost group (P = 0.017) (Figure 2).
Hazard ratios (HRs) of grade, HR, tumor size, and age in DFS were evaluated by univariate Cox proportional hazards regression model. In the univariate analysis of variables including grade (P = 0.41), hormone receptor (P = 0.09), size (P = 0.24), and age (P = 0.09), there was no significant role in local recurrence, but delivering radical dose by IORT had a significant role in occurring recurrence (P = 0.03) that could be explained by no recurrence in X-ray radical group and small size of our sample resulting in a probable error and it had not a true meaning. None of the variables had a significant role in recurrence in multivariate analysis.
5. Discussion
In this study, local recurrence was higher in the boost group of IORT in comparison with the EBRT, but it did not have a statistically significant difference. Even local recurrence in patients, who received a radical dose of IORT, in comparison with EBRT did not have a statistically significant difference, too. In separately analyzing beams, no significant difference in DFS was observed. Only we had a significant difference when we used an electron beam as a radical dose in comparison with a boost dose (P = 0.045). In this study, variables of grade, HR, size, and age had no significant role in DFS.
Local recurrence occurred in 8.8% (5 patients), 13.9% (5 patients), and 2.2% (1 patient) of the patients of the EBRT, boost, and radical groups, respectively. Of 5 patients in the boost group that showed recurrence, 3 were younger than 40 years, 2 had hormone receptor-negative tumors, 2 had a high-grade tumor, and 2 had tumors with 6 cm size. The patient in the radical group that showed recurrence had negative hormone receptor and high-grade tumor.
Some factors linked with the elevated rate of local recurrence in DCIS, like patients presenting with a palpable mass, are related to local recurrence more than those presenting with mammographic abnormality (4) or younger age in diagnosis (14), high grade, DCIS size (15), negative hormone receptor (16), and margin status (17). The higher incidence rate of DCIS in SEER analysis was because of mammography detection and most of them were non-comedo types (2). With this increasing number of patients with pure DCIS detected with screening mammography, doing radiotherapy by the IORT technique has a socio-economic benefit for patients and treatment systems. They can save their time and money and reduce the travel rate by this method.
IORT was not allowed for DCIS in the first guideline of the American Society for Radiation Oncology. However, in 2017 revision, it was allowed for mammography-detected cases and low- or intermediate-grade DCIS of ≤ 2.5 cm with the surgical margins of ≥ 3 mm. Concerning the patients with and without the history of receiving radiation, the former group was meaningfully less likely to experience the repetition of BCS for recurrence status. The women with the history of receiving radiation after surgery, at the initial time of DCIS diagnosis, experience more complications following surgery for recurrence (18).
We can preserve breast in local recurrence of those patients that received IORT in their first surgery with less experience of surgery complications.
So, as an appropriate alternative, IORT will deliver the secure dose of radiotherapy and preserve BCS if local recurrence happens.
5.1. Conclusions
IORT is a good alternative for WBRT in patients with DCIS because of its non-inferiority results in comparison with EBRT, its convenience for patients, and its protection of patients from EBRT complications. Besides, the patients need no long-lasting search for radiotherapy centers.
When patients receive IORT in their first surgery, if local recurrence occurs, it is possible to save the breast with IORT, and if mastectomy is required, it is possible to do skin-sparing mastectomy.
Being careful about age, tumor size, biological markers, and margin status is of high importance when using IORT for DCIS.