1. Background
The pivotal role of external-beam radiation therapy (EBRT) in the management of patients with localized prostate cancer (pCa) has been established (1-3). Strong evidence has supported the better clinical outcome of either higher total or per fraction radiation doses in the management of pCa (4-6). Originated from Brenner and Hall’s study, the positive effect of hypofractionated EBRT on the therapeutic ratio of localized pCa is demonstrated in several studies (7-9). So far, many large-scale randomized clinical trials have compared the clinical outcomes of hypofractionated versus conventional schedules (9-11).
Up to a few years ago, 3-dimensional conformal radiation therapy (3D-CRT) was the standard technology used in Iran to deliver this treatment. Intensity-modulated radiation therapy (IMRT) is a newer technology with higher radiation doses to target tissue, better dose conformity, and less toxicity of adjacent critical organs. Therefore, it enabled the Iranian physicians to apply hypofractionation schedules in the treatment of pCa. The clinical-oncology department of Shohada-e Tajrish Hospital is the leading in Iran that has utilized IMRT in the management of pCa.
2. Objectives
This retrospective cohort study, as one of the first reports from Iran, has compared the clinical outcomes of conventional and hypofractionated schedules of EBRT in patients with localized pCa.
3. Methods
3.1. Study Design, Participants, and Evaluation
This retrospective cohort study of the clinical outcomes of definitive EBRT in patients with pCa was carried out at the clinical-oncology department of Shohada-e-Tajrish Hospital. The study was approved by the ethical committee of Shahid Beheshti University of Medical Sciences (ethical code: IR.SBMU.MSP.REC.1398.846). Being a retrospective analysis of clinical outcomes of patients treated as per institutional protocol, informed consent clearance was waived by the Institutional Review Board. Case records of all patients with histologically-proven localized (stage: T1bN0M0-T4aN0M0) pCa treated with definitive EBRT in the department between April 2013 and March 2019 were analyzed. Patients with metastasis and a history of pelvic radiation or prostatectomy were excluded from the study. Demographic and clinical data were collected, including age at diagnosis, pretreatment prostate-specific antigen (PSA), Gleason score, and the clinical staging based on the American Joint Committee on Cancer (AJCC) 7th edition staging system (12). The patients were stratified to low-, intermediate-, and high-risk for biochemical failure based on the initial PSA, biopsy Gleason score, and clinical T stage according to the D’Amico et al.’s report (13). Upon risk stratification, the staging work-up was done based on the institutional guideline.
3.2. Treatment
In this section, we present the essentials of modality applied in our department in detail.
Before the simulation, gold seed fiducials implanted into the prostate apex under transrectal ultrasonography. After 2 weeks, planning computed tomography (CT) scans were taken with patients in the supine position with a comfortably full urinary bladder and an empty rectum. The same situation was observed during radiotherapy sessions. Segmentation was done as per the (European Organisation for Research and Treatment of Cancer) EORTC protocol and its updates (14-16). The clinical target volumes (CTVs) included prostate for low- to intermediate-risk patients and prostate with seminal vesicles for high-risk patients. The planning target volume (PTV) was generated by adding a 5 mm margin posteriorly and a 10 mm margin in other directions (17). Patients in the conventional group were treated with a total dose of 74 Gy (16 and 2 patients received 72 Gy and 76 Gy, respectively) at 2 Gy-fractions for 5 days per week using 3D-CRT via a 4-field technique. The Varian Clinac 600 C linear accelerator (Linac) with 6 megavoltage (MV) photon beams equipped with an 80-leaf Multi-leaf collimator (MLC) and the EclipseTM Treatment Planning System (Varian Medical Systems, Palo Alto, CA, USA) were used. The pelvic lymph nodes were electively irradiated in 45 patients, including 44 patients with high-risk and 1 patient with intermediate-risk disease. For these patients, the initial 45 Gy to the whole pelvis with 4 fields was followed by a cone down to the prostate and seminal vesicles. Others received an initial 45 Gy to the prostate and seminal vesicles followed by an additional 27 Gy to 31 Gy boost to the prostate plus a 1.5 cm margin via a 4-field technique. Plans were optimized to deliver the prescribed dose to more than 95% of PTV. Under the guidance of fiducials, daily target localization and alignment were applied, using the electronic portal imaging device (EPID).
Concerning patients in the hypofractionated group, for better delineation of the target, T2-weighted magnetic resonance images (using a Siemens Avanto 1.5 Tesla Magnetic resonance imaging (MRI) scanner, Erlangen, Germany) were fused with CT scans (using the Siemens Emotion System spiral 16-slice, Erlangen, Germany). The patients’ position, bowel and bladder preparation protocol, segmentation, and target volumes were defined the same as the conventional group, except for an 8 mm posterior margin of PTV (18). In contrast to the conventional group, none of the patients in the hypofractionated group received radiation to the pelvic lymph nodes. All patients were planned with a 9-field IMRT technique (0, 30, 60, 100, 150, 210, 270, 300, and 330°) delivering 70.2 Gy in 26 fractions, as the so-called hypofractionation regimen. A Varian Clinac 600 C Linac with 6 MV photon beams equipped with an 80-leaf MLC and the EclipsTM Treatment Planning System (Varian Medical Systems, Palo Alto, CA, USA) were used. All the plans were interactively optimized following our institutional planning protocol based on the study reported by Pollack et al. (5). The daily target localization and alignment were applied, using EPID of gold seed fiducials.
In combination with EBRT, intermediate- and high-risk patients received 6 and 36 months of androgen deprivation therapy (ADT), respectively. Four and 3 patients of intermediate- and high-risk groups received ADT for 9 and 24 months, instead.
During follow-up, the measurement of PSA was performed every 3 to 6 months after radiotherapy. Whole-body bone scintigraphy and CT scan were performed to detect distant metastases as necessary.
3.3. Endpoints
The primary endpoint of this study was to compare the biochemical or clinical relapse-free survival (bc-RFS) and overall survival (OS). To calculate these outcomes, we recorded the date of EBRT start, biochemical or clinical recurrence, and death or last follow-up. The biochemical and clinical control were defined, using the PHOENIX criteria (defined as a rise in PSA to nadir plus 2 ng/mL) and imaging studies (using pelvic MRI, bone scintigraphy, CT scan, or Prostate-specific membrane antigen (PSMA) scan), respectively (19). Times for bc-RFS and OS were calculated from the onset of definitive EBRT. Also, the biologically effective dose (BED) and equivalent dose (EQD2) were imported for a better comparison of two modalities.
3.4. Statistical Analysis
To summarize the data, we used frequencies/percentages and means/standard deviation (or medians and ranges) for categorical and continuous variables, respectively. We used the chi-square test of independence (or Fisher’s exact test) and independent-sample t-test to compare categorical and continuous variables, respectively. Also, we used the Kolmogorov-Smirnov test to determine the normal distribution of continuous variables.
Patients with localized pCa, who received definitive EBRT with conventional or hypofractionated schedules, were compared in terms of bc-RFS and OS. We used the Kaplan-Meier method to estimate the bc-RFS and OS and Cox’s model to present the bc-RFS and OS based on risk groups. All analyses were performed, using IBM SPSS Statistics version 26. The statistical significance level was set at 0.05.
4. Results
A total of 170 patients were included in the overall cohort. Eighty-one (47.7%) and 89 (52.3%) patients were in conventional and hypofractionated groups, respectively. The patients’ characteristics and comparable distribution of patients in either treatment group are shown in Table 1. The mean cumulative dose in terms of EQD2 to the prostate for conventional and hypofractionated groups was 72.9 Gy and 80.0 Gy, respectively. The median follow-up period for all patients was 42 months (range: 8 - 81 months). The median follow-up time for conventional and hypofractionated groups was 65 months (range: 8 - 81 months) and 34 months (range: 15 - 46 months), respectively. The difference in follow-up times was due to a more recent application of IMRT in our department. Therefore, we compared the 3-year bc-RFS and OS of 2 schedules in this study.
| Characteristics | Total (N = 170) | Conventional (N = 81) | Hypofractionated (N = 89) | P Value |
|---|---|---|---|---|
| Age at diagnosis, y | 0.41 | |||
| Values | 70.9 ± 6.9 | 71 ± 6.8 | 70.8 ± 7.1 | |
| Range | 53 - 88 | 53 - 88 | 54 - 88 | |
| Clinical stageb | 0.38 | |||
| T1 | 4 (0.02) | 1 (0.01) | 3 (0.03) | |
| T2 | 74 (0.44) | 33 (0.41) | 41 (0.46) | |
| T3 | 49 (0.29) | 18 (0.22) | 31 (0.35) | |
| T4 | 4 (0.02) | 3 (0.03) | 1 (0.01) | |
| Unknown | 39 (0.23) | 26 (0.33) | 13 (0.15) | |
| Gleason score | 0.95 | |||
| ≤ 6 | 56 (0.33) | 26 (0.32) | 30 (0.34) | |
| 7 | 55 (0.32) | 25 (0.31) | 30 (0.34) | |
| 8 - 10 | 56 (0.33) | 27 (0.33) | 29 (0.32) | |
| Unknown | 3 (0.02) | 3 (0.04) | 0 | |
| Pre-treatment PSA, ng/mL | 0.47 | |||
| ≤ 10 | 50 (0.30) | 22 (0.27) | 28 (0.31) | |
| 10.1 - 20 | 47 (0.27) | 20 (0.25) | 27 (0.30) | |
| > 20 | 72 (0.42) | 38 (0.47) | 34 (0.39) | |
| Unknown | 1 (0.01) | 1 (0.01) | 0 | |
| Risk stratificationc | 0.49 | |||
| Low-risk | 10 (0.06) | 5 (0.06) | 5 (0.05) | |
| Intermediate-risk | 58 (0.34) | 24 (0.30) | 34 (0.39) | |
| High-risk | 102 (0.60) | 52 (0.64) | 50 (0.56) | |
| Duration of ADT, mo | 0.28 | |||
| Not received | 10 (0.06) | 5 (0.06) | 5 (0.05) | |
| 6 | 53 (0.31) | 24 (0.30) | 29 (0.32) | |
| 9 | 5 (0.03) | 1 (0.01) | 4 (0.05) | |
| 24 | 3 (0.02) | 0 (0.00) | 3 (0.03) | |
| 36 | 99 (0.58) | 51 (0.63) | 48 (0.55) |
. Clinical Characteristics of the Patient Cohorta
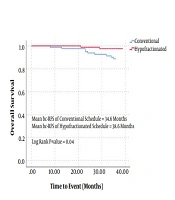
Figure 1 demonstrates the comparison between conventional and hypofractionated groups in terms of bc-RFS and OS. The mean bc-RFS of patients in conventional and hypofractionated groups was 34.9 and 35.4 months, respectively (CI 95%: 34.5 - 35.7, P = 0.25). Among them, 2 (2%) and 1 (1%) patients developed local recurrence, while, 11 (13%) and 2 (2%) patients developed distant recurrence in conventional and hypofractionated groups, respectively. Accordingly, the mean OS of patients in conventional and hypofractionated groups was 34.6 and 38.6 months, respectively (CI 95%: 37.3 - 38.6, P = 0.04). In the conventional group, there were 17 deaths. The causes of deaths were metastatic pCa in 8 cases, cardiac disease in 4 cases, cerebrovascular accident in 3 cases, cirrhosis in 1 case, and car accident in 1 case. In the hypofractionated group, there were 2 and 1 deaths due to cardiac disease and metastasis, respectively. In comparison with conventional schedule, patients treated with hypofractionation had a trend to longer (38.7 vs 35.1 months) 3-year cancer-specific survival (CI 95%: 37.8 - 38.8, P = 0.66).
We also investigated details about the risk subgroups. Table 2 summarizes the clinical outcomes of risk groups based on the EBRT schedule. It shows that the effect of hypofractionation on improving the OS approached the borderline of significance for intermediate- and high-risk pCa (CI 95%: 0.02 - 1.46, P = 0.054). However, this finding was not met for bc-RFS (CI 95%: 0.13 - 1.90, P = 0.28).
| Conventional Schedule, % | Hypofractionated Schedule, % | P Value | |
|---|---|---|---|
| 3-year bc-RFS | 0.287 | ||
| Low-risk | 100 | 100 | |
| Intermediate-risk | 100 | 100 | |
| High-risk | 90.3 | 92 | |
| Total | 92.5 | 96.6 | |
| 3-year OS | 0.054 | ||
| Low-risk | 100 | 100 | |
| Intermediate-risk | 95.8 | 100 | |
| High-risk | 84.6 | 96 | |
| Total | 88.9 | 97.8 |
Three-Year Biochemical or Clinical Relapse-Free Survival and Overall Survival Based on Risk Groups
5. Discussion
In this study, both treatment groups shared common conditions in terms of patients’ clinical characteristics, target definition, position-reiterating modality, and duration of ADT. We observed that 3-year OS was significantly higher in our patients treated with hypofractionated radiotherapy using IMRT. In subgroup analysis, we showed that the positive effect of hypofractionation on a 3-year OS was limited to patients with intermediate- to high-risk localized pCa. This finding may provide the answer to the simple question of whether hypofractionation has a clinical advantage over conventional fractionation in the definitive management of pCa. In Table 2, we demonstrated the almost significant positive effect of hypofractionated schedule on the OS of patients with intermediate- and high-risk pCa; however, in contrast to the conventional group, they did not receive radiation to the pelvic nodes. This may highlight the crucial role of ADT in patients with intermediate- and high-risk pCa.
So far, many clinical trials were conducted to test the value of hypofractionated EBRT on pCa. A large phase III randomized clinical trial by Dearnaley et al. (10) (CHHiP trial) showed the non-inferiority of hypofractionated (60 Gy in 20 fractions over 4 weeks) versus conventional (74 Gy in 37 fractions over 7.4 weeks) EBRT for localized pCa in terms of OS and bc-RFS. Another phase III randomized trial by Arcangeli et al. (9) demonstrated similar results in a 10-year follow-up. Although different in radiotherapy schedules, our finding is comparable to these clinical trials.
In 2015, Kalbasi et al. (20) have shown that dose-escalated EBRT can improve the survival of patients with intermediate- and high-risk pCa, but not low-risk group. In the present study, we evaluated the effect of higher BED of the prostate gland in different risk groups. In this study, we approached the higher BED by larger fractions (i.e. the hypofractionated program using IMRT). In comparison with conventional fractionation, the OS of patients with intermediate to high-risk pCa had tended to improve with hypofractionated EBRT. However, a meta-analysis by Guo et al. (21) showed the comparable clinical outcomes of hypofractionation and conventional EBRT in intermediate- to high-risk localized pCa.
The limitations of the present study should be considered before interpreting the results. Firstly, the definition of failure-free survival was based on biochemical or clinical findings for relapse. Hence, there might be some cases that were missed due to reliance merely on clinical evidence for relapse. Secondly, the shorter follow-up of patients in the hypofractionation group limited the comparative analysis to 3 years of follow-up. Due to the slow-growing nature of pCa, long-term follow-up may increase the rate of recurrence or even mortality. Thirdly, our study did not include the results for toxicities. To better compare the two modalities, one should include the comparative rectal and genitourinary toxicities. Fourthly, the small sample size could have biased the results. Fifthly, the retrospective analysis of medical records made poor control on covariates of recurrence and survival. To resolve these critical issues, larger prospective studies are necessary.
5.1. Conclusions
In sum, we found that hypofractionated EBRT was associated with an improved OS for men with intermediate- and high-risk, but not low-risk, pCa. This clinical benefit was not demonstrated for bc-RFS. However, due to the limitations of our study, these findings should be utilized cautiously when directed in clinical treatment. Large well-designed clinical trials are required to reveal our notion.