1. Background
With an overall incidence of about 1 to 2 in 100 000 person-year in children, acute lymphoblastic leukemia (ALL) is the most frequent form of malignant neoplasia diagnosed in ages 0 to 14 years old (1, 2). Notwithstanding copious studies to improve disease outcomes, efforts have not yet converted into a better prospect and bone marrow relapse is still the leading cause of person-year of life lost in this malignancy (3). Although risk-adapted chemotherapy is conventionally accepted as an efficient therapeutic approach in pediatric with ALL in Iran, some of the patients, who are not considered ‘high-risk’ and treated accordingly, would relapse after initially successful treatment with an approximate morbidity and mortality rate of 58% (4). Adding to this complexity, tumor lysis syndrome (TLS) is another severe and life-threatening complication that contributes to progress disease toward an unfavorable outcome, at least partly, through the electrolyte and metabolic disturbances leading to cardiac arrhythmia, seizure, multi-organ failure, and eventually death (5).
Despite the evidence, attest stating the survival analysis retains a specific clinical and biological significance in patients with ALL (6), an important challenge relevant to these studies is the fact that “time to relapse” is susceptible to bias. As the death of some participants occurs before relapse, time to relapse cannot be evaluated accurately due to inappropriate estimation of the risk of relapse; an event which is known as competing risk, in which the occurrence of one event masks the occurrence of another (7). Taking advantage of these facts, it is reasonable to assume that treating the competing events as censored observation and using the Kaplan-Meier method is an erroneous and inappropriate estimate in this setting. Nevertheless, cumulative incidence function (CIF) could correctly predict the chance of relapse and death (8). The competing risks have been considered in several medical conditions, such as nephrology (9) and cardiologic diseases (10). The disease-free survival rate of ALL is reported completely differently depending on the countries; 54.9 to 59.2% in developed countries (11) vs. 30 to 40 and 57% in developing countries (12) and Iran (13), respectively.
2. Objectives
The present study was designed to identify the associated risk factors for relapse and non-related mortality (NRM) - mortalities that were not related to ALL for pediatric patients with ALL in standard - and high-risk group patients. This was done, using a newly-developed competing risk model. Finally, the predictability of the fitted model was assessed.
3. Methods
3.1. Study Sample
Children aged ≤ 16 years with the diagnosis of ALL, who were referred to Sheikh Hospital, Mashhad, Iran from 2007 to 2016 were enrolled in the study. The study protocol was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1397.1284). Informed consent was taken from the patient’s parents. Children who were treated by standard Berlin-Frankfurt-Munster 2009 (BFM-2009) (14) chemotherapy regimen were included in the study.
Relapse was considered the primary event of interest and NRM was the competing risk (secondary event of interest). Age, gender, serum levels of white blood cell (WBC), hemoglobin (Hb), Platelet (< 150000 vs. > 150000), lactic acid dehydrogenase (LDH < 516 vs. > 516), hemorrhagic, and clinical characteristics of the patients, such as the presence of rheumatoid signs, TLS, hepatosplenomegaly, lymphadenopathy, the involved cell line (T vs. B), and central nervous system (CNS) disease were considered. Patients were categorized into the high-risk group when the patient’s age was lower than 1 year, WBC count was > 50,000/µL, the cell line was T cell, and CNS involvement was present, otherwise were categorized into the low-risk group (15). Relapse, defined as any case of development of disease recurrence after treatment, was diagnosed via morphologic analysis of bone marrow aspiration (16).
3.2. Improper Form of Two-Parameter Weibull Model
The time from ALL diagnosis to the occurrence of relapse or NRM is considered t. Otherwise, the end of the study is considered t (censored patients). CIF for relapse and mortality are as follows:
Where
4. Results
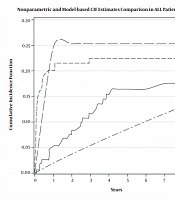
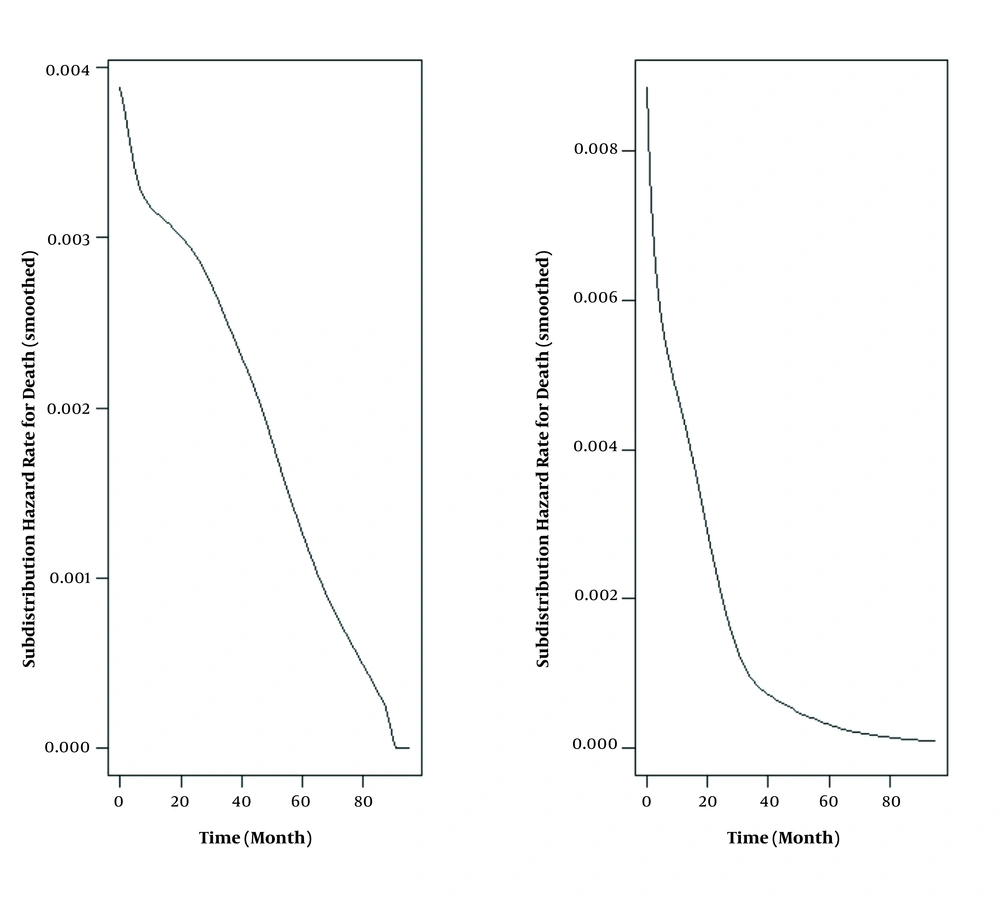
Table 1 shows the demographic and clinical characteristics of the 424 analyzed patients with a mean age of 5.56 ± 3.75 years, from whom 172 (40%) were female. Median (IQR) follow-up time was 43.29 (0.033, 96) months; 45 (10.6%) patients had relapse, 73 (17.2%) had NRM, and 306 (72.1%) survived until the final follow-up. Relapse-free survival rates at 1, 3, and 5 years were 97, 91, and 88%, and overall survival rates were 86, 83, and 82%, respectively. The decreasing trend in the hazard of relapse and death is clear in Figure 1.
| Factor | Values |
|---|---|
| Female | 172 (40) |
| High-risk group | 154 (36) |
| TLS | 21 (5) |
| LDH < 516 U/L | 127 (30) |
| CNS disease | 13 (3) |
| Initial WBC< 10000/µL | 259 (61) |
| Initial WBC 10000 - 50000/µL | 103 (24.5) |
| Initial WBC 50000 - 100000/µL | 27 (6.5) |
| Initial WBC > 100000/µL | 35 (8) |
| Platelet < 150000/L | 339 (80) |
| Hepatosplenomegaly | 178 (42) |
| Hemorrhage | 59 (14) |
| Rheumatoid signs | 133 (31) |
| Lymphadenopathy | 87 (20) |
| B cell involvement | 29 (7) |
| Age (y) | 5.56 ± 3.75 |
| Hemoglobin (mg/dL) | 8.02 ± 2.55 |
| LDH (U/L) | 1482 ± 2460 |
Patient’s Demographic and Clinical Factors a
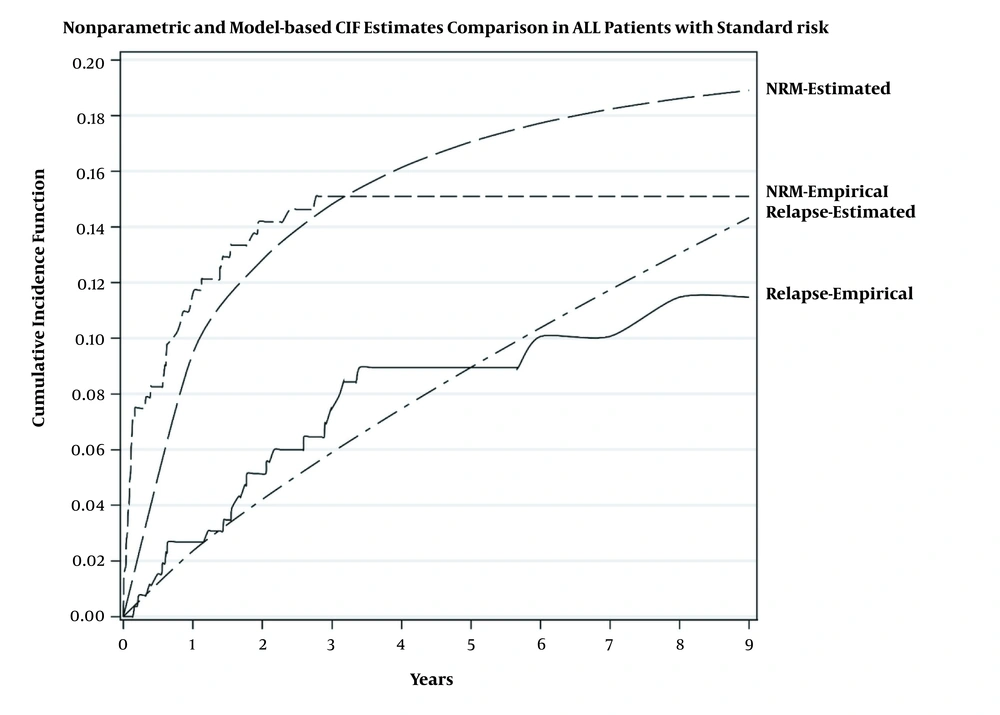
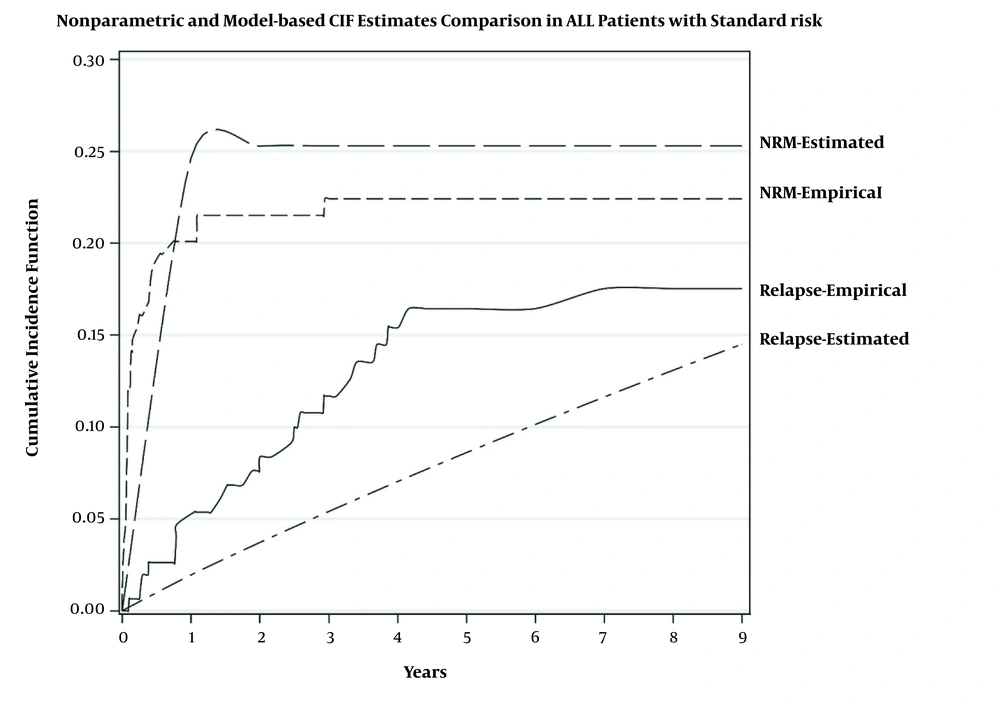
Table 2 shows that in the standard-risk group, TLS significantly increased either the relapse risk (HR of 13.47, 2.05 - 67.54) or mortality risk (HR of 19.57, 2.24 - 32.18). In the high-risk group, the higher level of hemoglobin (HR of 1.25, 1.02 - 1.53), platelet (HR of 4.50, 1.58 - 1.97), and LDH (HR of 4.77, 1.40 - 16.38) were significantly associated with higher relapse risk (Table 3). In addition, the mortality risk in patients with TLS was 4.96 (1.53 - 14.27) times that of the patients without TLS. Figures 2 and 3 show the estimation of relapse and NRM incidence rate (estimated) and observed incidence rate (empirical) for standard- and high-risk groups. The figures show that the fitted model had better predictability for patients in the standard-risk group in comparison to the patients in the high-risk group. The proportional hazard assumption for the model was checked and it was satisfied.
| Factor | Relapse | NRM |
|---|---|---|
| GenderFemale | 0.58 (0.23 - 1.44) | 0.21 (0.06 - 0.71) |
| Hemoglobin | 0.97 (0.84 - 1.13) | 0.92 (0.72 - 1.18) |
| Platelet | 1.90 (0.72 - 4.96) | 0.55 (0.20 - 1.49) |
| LDH | 0.89 (0.36 - 2.26) | 2.64 (0.61 - 11.94) |
| Hemorrhagic | 2 (0.36 - 2.26) | 1.22 (0.16 - 6.65) |
| Rheumatoid signs | 1.06 (0.70 - 5.5) | 1.07 (0.25 - 4.36) |
| TLS | 13.47 (2.05- 87.54) | 39.57 (2.24 - 332.18) |
| Hepatosplenomegaly | 1.07 (0.43- 2.58) | 1.92 (0.69 - 5.83) |
| Lymphadenopathy | 0.53 (0.14- 2.17) | 0.31 (0.07 - 1.29) |
The Hazard Ratio (95% HPD Region) of the Factors on the Risk of Relapse and Mortality-Standard Risk a
| Factor | Relapse | NRM |
|---|---|---|
| Gender | 0.95 (0.39 - 2.23) | 1.82 (0.61 - 5.20) |
| Hemoglobin | 1.25 (1.02 - 1.53) | 0.85 (0.73 - 1.01) |
| Platelet | 4.50 (1.58 - 1.97) | 0.65 (0.07 - 3.88) |
| LDH | 4.77 (1.40 - 16.38) | 1.27 (0.37 - 4.54) |
| Hemorrhagic | 0.45 (0.11 - 2) | 0.14 (0.05 - 0.42) |
| Rheumatoid signs | 0.73 (0.26 - 2.26) | 0.80 (0.24 - 2.33) |
| TLS | 0.95 (0.24 - 3.40) | 4.96 (1.53 - 14.27) |
| Hepatosplenomegaly | 0.52 (0.21 - 1.36) | 0.86 (0.37 - 1.94) |
| Lymphadenopathy | 0.42 (0.15 - 1.28) | 1.25 (0.44 - 3.65) |
The Hazard Ratio (95% HPD Region) of the Factors on the Risk of Relapse and Mortality-High-Risk a
5. Discussion
Being one of the most common malignancies affecting children, ALL has attracted tremendous attention. In a study conducted by Mehravar et al., it has been reported that out of 216 children suffering from acute leukemia who have been referred to MAHAK’s Pediatric Cancer Treatment and Research Center from 2007 to 2011, nearly 80% had ALL with the mean age of 0.92 ± 5.5 years (18). Albeit by matching the number of deaths to the total number of cases, one may conclude that most of the patients with ALL are recovering, taking a look at the mortality incidence of this malignancy remind us that ALL is still taking its toll. Precise inspection of pertinent literature disclosed that the incidence of relapse and the mortality rate of the diseases is different from one study to another (10, 11, 13). While Pege et al. reported that only 23% of the patients with ALL survived for 5 years (19), the results of a study conducted by El-Ghammaz et al. demonstrated an outrageous risk of death, reporting the disease mortality rate up to 25.6% (20).
Although the diverse health care systems and medical facilities together with different socio-economic conditions of countries may explain, at least partly, this diversity, it is worth noting that utilization of inappropriate methods to estimate the risk of relapse would also contribute to the proclamation of heterogeneous data reflecting relapse and mortality incidence rates in ALL. Given these controversies, we aimed at estimating the overall survival of pediatric ALL patients who aged less than 16 years old. The results of this study showed that the 5-year survival rate for ALL patients was 82% and the disease-free survival rate was 88%. This finding was in contrast to another study conducted on Iranian ALL patients, which reported a 5-year survival of 57% for patients (13). This discrepancy could be due to several interfering factors that could define the survival of the patients.
It has been reported that the emergence of TLS, which mainly occurs after the initiation of chemotherapy, is a common complication in ALL patients and is significantly associated with in-hospital mortality (21). Given this, it was tempting to investigate whether our newly-developed competing risk model could predict the plausible correlation between TLS occurrence and disease relapse. The results of the present research showed that the risk of relapse and mortality was higher in pediatrics with a history of TLS compared to other groups. We found that the risk of relapse in the patients with the experience of TLS was 4 times higher than even the high-risk group. In agreement with our results, another study declared that there is a significant correlation between the occurrence of TLS in acute myeloid leukemia patients and the higher mortality rate (22), suggesting that probably this indicator could be served as a prognostic factor for patients with leukemia. It is noteworthy due to the low prevalence of TLS in the present population. That is why a wide confidence interval is provided. However, in a different population, the incidence of TLS in patients with leukemia is reported variable including 14, 4, and 2% (23).
Another parameter, which has been claimed to be involved in the occurrence of disease recurrence is gender. For a long time, it has been suggested that the response of cancer patients to conventional treatment strategies is quite different between males and females (24). Although the majority of studies have suggested that the male gender is an indicator of worse survival and risk of disease recurrence in both solid and hematological malignancies (25), in some cases there are some controversies. In a study conducted on urothelial carcinoma of the bladder (UCB), it has been reported that women are at higher risk for disease recurrence after local treatment compared with male patients (26). Moreover, the results of another study failed to report any significance between the survival rate in female and male patients with acute leukemia (27). Of note, in the present study, we found that girls with ALL were at a lower risk of disease relapse compared to boys. This could be due to the participation of sex hormones in the determination of cancer cells' response to chemotherapeutic drugs (28).
Apart from the genetics architecture, which could define the outcome of cancer patients, the current cancer management approaches have focused on the indicators, which could be evaluated more conveniently without any complication for patients. Given this, intense attention has been attracted to the biochemical parameters. In a study performed by Allott et al., it has been demonstrated that serum lipid profile level in prostate cancer patients could be considered to be a valuable prognostic factor, as the elevation in the levels of serum lipids had a remarkable association with disease relapse (29). Moreover, TG/HDL-C ratio (THR) has been introduced as a predictor of poor prognosis in breast cancer patients (30). Although several early indicators have been reported to be associated with the prognosis of acute leukemia (31), little is known about the parameters, which could determine the extent of relapse incidence in high-risk groups. The results of the present study reported for the first time a correlation between the incidence of disease relapse and some laboratory indexes, such as hemoglobin level, platelet count, and LDH in the high-risk group. We reported that in the high-risk group, patients with higher hemoglobin, platelet, and LDH were more likely to experience relapse.
To sum up, the present study suggests different factors for predicting the risk of disease relapse as well as mortality more accurately in pediatric patients with ALL such as TLS. However, further evaluation on the larger population of patients with ALL is demanded to ascertain the precision of such parameters in leukemic management strategies.