1. Background
Nitrogen-containing bisphosphonate compounds bind to osteoclasts and suppress the mevalonate pathway, resulting in inhibition of osteoclast function and thereby bone resorption (1). Zoledronic acid (ZA) is the most frequently used intravenous (IV) bisphosphonate with multiple clinical applications (2). It has been approved for the treatment of postmenopausal osteoporosis (3) and Paget’s disease of bone (4). ZA has also many clinical applications in the oncology field. These include treatment of metastatic bone diseases (5), malignancy-related hypercalcemia (6), cancer therapy-induced bone loss (7), and multiple myeloma (8).
More than 90% of patients with cancer at advanced stages might have bone metastasis (9, 10). As a result, patients may suffer from skeletal-related events (SRE) such as hypercalcemia, bone fracture, severe bone pain, and spinal cord compression (11). Administration of IV ZA in patients with breast and prostate cancers that are metastatic to bone caused a significant reduction in bone pain and other SRE. In addition, ZA increased bone mineral density and patients’ overall survival (5, 12-18). ZA also demonstrated positive effects in patients with cancers that are less metastatic to bone including advanced lung, thyroid, and renal cell carcinoma (19-21).
A retrospective analysis of 256 patients with cancer who received ZA, demonstrated occurrence of severe adverse effects including: hypocalcemia (n = 22, 8.5%), renal dysfunction (n = 19, 7.4%), jaw osteonecrosis (n = 4, 1.5%), and symptomatic hypocalcemia (n = 2, 0.7%) (22). Conversely, results from one retrospective study among breast cancer patients with bone metastasis showed that such events rarely encountered with ZA administration (23). ZA infusion also resulted in atrial fibrillation in postmenopausal osteoporosis patients (24). However, most of ZA adverse effects were described as acute phase responses (25). These mainly included; pyrexia, musculoskeletal, gastrointestinal (GI), eye inflammation, and other general adverse effects (3, 25, 26). Additionally, ZA treatment resulted in significant changes in biochemical parameters such as serum calcium, phosphorus, and creatinine levels (23, 24, 27).
The positive therapeutic outcomes of ZA in patients with cancer necessitates encouraging its use among these patients. Minimal or absence of major adverse effects with ZA would likely add more valuable recommendations for ZA prescription and improve patients’ compliance. Genetic variations among different ethnicities may however contribute to variation in drug adverse effects profile in different populations (28). Accordingly, it is prudent to investigate the adverse effect profile of ZA among various clinical settings in populations. To the best of our knowledge, evaluation of different aspects of ZA safety profile as well as its effects on biochemical parameters among patients with cancer has not been studied in Jordan.
2. Objectives
This study aimed at (1) evaluating the adverse effects of ZA among patients with cancer and (2) assessing ZA effects on the serum levels of different biochemical parameters related to ZA toxicities.
3. Methods
3.1. Study Design and Subjects
This retrospective cohort study was conducted among patients with cancer receiving IV infusion of ZA (Zometa) at the chemotherapeutic unit in King Abdullah University Hospital (KAUH)/ Jordan. The study was approved by the Research Committee and the Institutional Review Board (IRB) of Jordan University of Science and Technology (JUST)/Jordan (IRB approval no. 9/118/2018). Written informed consent was obtained from all subjects before being interviewed at the chemotherapeutic unit. The total number of patients who received ZA and enrolled in this study was 98 patients during October 2018 to December 2019.
3.2. ZA Adverse Effects
Patients were interviewed to assess whether they have experienced ZA related symptoms, including acute events such as myalgia, bone pain, influenza-like symptoms, headache, pyrexia, gastrointestinal (GI) symptoms (nausea, vomiting, diarrhea, abdominal pain, and heartburn), visual, and hearing symptoms as well as serious adverse effects (jaw osteonecrosis, cardiac and renal problems, or allergic reactions). Patients were characterized based on their age, gender, smoking status, cancer type, indications for ZA infusion, frequency of infusions, and a number of ZA cycles. After the interview, the electronic medical records of the enrolled patients were retrospectively assessed to confirm the documentation of adverse effects reported by these patients.
3.3. ZA Adverse Effects with Multiple Infusions
In order to assess the frequency of ZA adverse effects with repeated infusions, patients who were treated with ZA for more than 6 cycles (n = 44 patients), were evaluated regarding the occurrence of ZA adverse effects after the first, second to sixth, and more than six infusions.
3.4. Effects of ZA on Biochemical Parameters
The effects of ZA treatment on the serum levels of different biochemical parameters were retrospectively assessed by checking patients’ electronic medical records. These include calcium, phosphorus, sodium, potassium, magnesium, total protein, creatinine, and urea. The clinical laboratory testing for the aforementioned biochemical parameters was done according to the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Levels were evaluated at 3 occasions: first, baseline records before the first infusion of ZA (pre-treatment); second, post-treatment of first infusion/before the second infusion; and third, the final level at the date of patient’s interview.
Statistical analysis was performed using GraphPad software (GraphPad prism, Prism8 for windows, version 8). Data on biochemical parameters were expressed as mean ± standard deviation. The Paired t-test was used to compare data between the first and the second readings, as well as between the first and the third readings. The P value less than 0.05 was considered as statistically significant.
4. Results
4.1. Patients’ Characteristics
In this study, 98 patients with cancer who have been prescribed ZA (Zometa) were interviewed at the chemotherapeutic unit of KAUH. The characteristics of these patients are summarized in Table 1. Of these patients, 9 were male, and 89 were female. The median age was 56.5 years (age range = 33 - 82 years). Among these patients, 12 were smokers at the time of the interview, while 86 patients were non-smokers.
| Patients Characteristics | Values |
|---|---|
| Gender | |
| Female | 89 (90.8) |
| Male | 9 (9.2) |
| Age, y | 56.5 (33 - 82) |
| Smoking status | |
| Smoker | 12 (12.2) |
| Non-smoker | 86 (87.8) |
| Primary cancer type | |
| Breast | 82 (83.7) |
| Prostate | 6 (6.1) |
| Multiple myeloma | 4 (4.1) |
| Liver | 3 (3.1) |
| Bone metastasis with unknown primary | 2 (2) |
| Ovarian | 1 (1) |
| Indication for ZA infusion | |
| Bone metastasis | 66 (67.3) |
| Cancer therapy induced osteoporosis | 32 (32.7) |
| Frequency of ZA infusions | |
| Every 1 month | 66 (67.3) |
| Every 6 months | 32 (32.7) |
| Number of ZA cycles | 5.5 (1 - 36) |
Characteristics of the Patients Demographics, Cancer Types, and Intravenous ZA Treatment among Patients with Cancer (Total Number of Patients = 98)a
Regarding ZA treatment, the distribution of patients who received ZA consisted of breast cancer (n = 82), prostate cancer (n = 6), multiple myeloma (n = 4), liver cancer (n = 3), bone metastasis with unknown primary (n = 2), and ovarian cancer (n = 1). ZA was indicated for 66 patients due to bone metastasis and 32 patients due to cancer therapy-induced osteoporosis. ZA was administered in 4 mg doses within 100 mL 5% dextrose for a period of 30 minutes as an IV infusion once/month for 66 patients (bone metastasis and multiple myeloma), while 32 patients received ZA every 6 months (cancer therapy-induced osteoporosis). The median for the number of received infusion cycles was 5.5 cycles (range = 1 - 36 cycles).
4.2. ZA Adverse Effects
The adverse effects recorded in patients with cancer who received ZA are summarized in Table 2. The most common adverse effects were acute events including myalgia (48%), bone pain (36.7%), influenza-like symptoms (34.7%), headache (31.6%), and pyrexia (22.45%). In addition, patients developed GI problems including nausea (21.4%), vomiting (10.21%), diarrhea (10.21%), heartburn (7.14%), and abdominal pain (6.12%). Symptoms related to eye were reported by 14.3% of the patients (redness, pain, and a single case of cataracts). Hearing problems such as tinnitus and hyperacusis were reported by 13.3% of patients. Most of the aforementioned symptoms were self-limiting without the need for medical intervention. However, antipyretics were prescribed to treat pyrexia and influenza-like symptoms. Further, pain killers (acetaminophen/ paracetamol) were prescribed for management of myalgia, bone pain, and headache in mild-moderate cases. Tramadol was prescribed in limited cases of severe bone pain and myalgia. All patients reported that GI problems were tolerated without the need for medications. Patients who complained of eye symptoms were referred for ophthalmologic consultation.
| Adverse Effects | Values |
|---|---|
| Myalgia | 47 (47.96) |
| Bone pain | 36 (36.73) |
| Influenza like symptoms | 34 (34.69) |
| Headache | 31 (31.63) |
| Pyrexia | 22 (22.45) |
| Eye symptoms | 14 (14.29) |
| Hearing symptoms | 13 (13.27) |
| Nausea | 21 (21.43) |
| Vomiting | 10 (10.21) |
| Diarrhea | 10 (10.21) |
| Heartburn | 7 (7.14) |
| Abdominal pain | 6 (6.12) |
Zoledronic Acid Adverse Effects among Patients with Cancer (Total Number of Patients = 98)a
Serious ZA adverse effects such as jaw osteonecrosis, cardiac, renal or allergic problems were not reported.
4.3. ZA Adverse Effects with Multiple Infusions
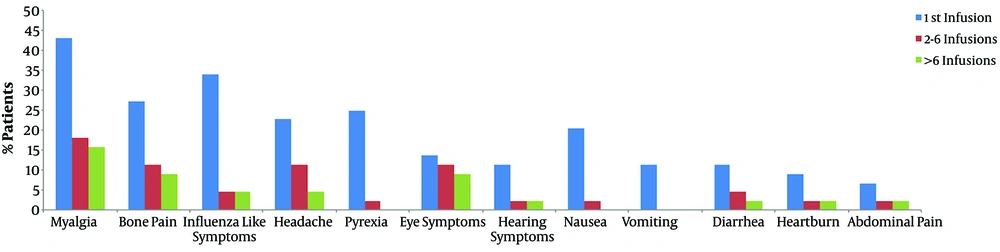
The occurrence of ZA adverse effects with multiple infusions was evaluated among patients who were treated with ZA for more than 6 cycles (n = 44 patients). Figure 1 shows the percentage of patients who experienced ZA adverse effects after the first, second to sixth, and more than six infusions. It was noticed that the frequency of these symptoms was at its highest percentage after the administration of the first infusion and decreased over time with repeated infusions. The decline was less pronounced with eye symptoms.
The frequency of Zoledronic acid adverse effects with repeated infusions. Columns from left to right show the frequency for zoledronic acid adverse effects for the first (1st), second to sixth (2 - 6) and more than six (> 6) infusions. Analysis was done among patients received more than six infusions (n = 44).
4.4. ZA Effect on Biochemical Parameters
Serum levels of different biochemical parameters were retrospectively reviewed from patients’ medical records as summarized in Table 3. Small, but statistically significant (P < 0.0001), reduction of calcium levels, was observed after the first infusion. However, no statistically significant difference was observed between pre-treatment and final serum calcium (P = 0.636). There was also no statistically significant difference in levels of other serum electrolytes including phosphorus, sodium, potassium, and magnesium between the pre-treatment and after the first infusion (P ≥ 0.27) as well as to the final treatment (P ≥ 0.075), except for potassium, where the significant reduction was observed upon the comparison of final treatment with pre-treatment levels (P = 0.003). Regarding creatinine, a significant reduction was observed between pre-treatment and after the first infusion (P = 0.031). However, elevation in creatinine levels was observed in the final reading compared to pre-treatment, although it was statistically insignificant (P = 0.059). No statistically significant difference was found in serum urea level between infusion groups. Alternatively, the administration of the first infusion resulted in a significant reduction in total protein level (P = 0.008), but no significant difference was found in the final reading (P = 0.577). Notably, all readings of different infusions were within the normal levels for all evaluated parameters.
| Biochemical Parameter, Unit | Normal Serum Levelb | Pre-Treatment (Baseline) | After the First Infusion/Before the Second Infusion | P Value | Pre-Treatment (Baseline) | At Date of Interview (Final Level) | P Value | Available Data |
|---|---|---|---|---|---|---|---|---|
| Calcium, mmol/L | 2.20 - 2.55 | 2.38 ± 0.12 | 2.31 ± 0.13 | < 0.0001 | 2.38 ± 0.12 | 2.39 ± 0.12 | 0.636 | 96 |
| Phosphorus, mmol/L | 0.81 - 1.45 | 1.19 ± 0.23 | 1.18 ± 0.78 | 0.889 | 1.19 ± 0.23 | 1.15 ± 0.21 | 0.147 | 89 |
| Sodium, mmol/L | 135 - 153 | 140.06 ± 3.02 | 140.33 ± 3.22 | 0.470 | 140.06 ± 3.02 | 140.12 ± 2.93 | 0.860 | 97 |
| Potassium, mmol/L | 3.70 - 5.40 | 4.43 ± 0.45 | 4.38 ± 0.46 | 0.400 | 4.43 ± 0.45 | 4.27 ± 0.38 | 0.003 | 97 |
| Magnesium, mmol/L | 0.66 - 0.99 | 0.83 ± 0.1 | 0.81 ± 0.1 | 0.271 | 0.83 ± 0.1 | 0.81 ± 0.1 | 0.075 | 93 |
| Total protein, g/L | 64 - 83 | 73.58 ± 7.17 | 71.55 ± 5.90 | 0.008 | 73.58 ± 7.17 | 73.12 ± 6.70 | 0.577 | 94 |
| Creatinine, mmol/L | 62 - 115 | 62.25 ± 14.23 | 59.86 ± 14.41 | 0.031 | 62.25 ± 14.23 | 65.30 ± 22.50 | 0.059 | 96 |
| Urea, mmol/L | 2.86 - 8.21 | 4.86 ± 1.91 | 4.66 ± 1.73 | 0.290 | 4.86 ± 1.91 | 4.85 ± 1.73 | 0.965 | 96 |
Serum Levels of Biochemical Parameters Before and After Zoledronic Acid Intravenous Infusions among Patients with Cancera
5. Discussion
With intent to assess the adverse effects profile of ZA among patients with cancer, we interviewed patients who received ZA IV infusions for treatment of bone metastasis (67%) and cancer therapy-induced osteoporosis (33%). ZA treatment is recommended for the well-established beneficial effects; however, major adverse effects may affect the patient’s compliance toward treatment (29). Upon assessment of ZA adverse effects, acute events including myalgia, bone pain, influenza-like symptoms, headache, and pyrexia were identified. These are in accordance with findings from previous studies on the safety profile of ZA among oncology and osteoporosis patients (25, 30). The underlying mechanisms contributing to the previous adverse effects are not fully understood. However, it has been suggested to be caused by the increased levels of inflammatory mediators such as interleukin-6 and tumor necrosis factor-α as a result of gamma delta T cells activation (31). Notably, according to interviewed patients, these adverse effects were self-limiting, tolerable, and/or resolved with symptomatic treatment. Since patients’ compliance was not affected, no further investigation was committed.
ZA was reported to be associated with increased risk of jaw osteonecrosis (22), a rare but significantly incapacitating adverse effect. Jaw osteonecrosis was not reported in the current study. This is in agreement with results from other studies that investigated the use of ZA for breast cancer patients with bone metastasis and female patients with osteoporosis (23, 26). This finding might be attributed to the meticulous oral and dental examination of patients at KAUH to assess any pre-existing condition that may require surgical dental treatment following ZA treatment which may result in jaw osteonecrosis. Our records have shown that patients have maintained good oral hygiene as well as performed required dental and periodontal prophylactic treatments before and during ZA therapy.
ZA’ association with renal toxicity was previously reported in other studies and was correlated with doses exceeding 4 mg given over less than 15 minutes of administration time (32). In the current study, ZA was administered in 4 mg doses within 100 ml 5% dextrose for a period of 30 minutes. Although no renal impairment was documented for any of the patients in this study, medical problems that might increase the risk of renal dysfunction should be considered carefully when prescribing bisphosphonates, especially diabetes mellitus, hypertension, advanced age and on medications that may have negative impact on renal functions (23). In addition, evaluation of renal function should be routinely performed for all patients undergoing ZA treatment with sufficient hydration being highly recommended (23).
Although cardiac-related adverse effects such as atrial fibrillation were reported in female patients with osteoporosis (24), the subsequent studies described no effects of ZA on cardiac rhythm (26), which was also observed among the patients of the current study. In addition, allergic reactions or infusion site reactions such as erythema, pruritis, or pain were not reported, which supports previous studies (26).
In this study, we reported that the frequency of ZA acute events was reduced with repeated infusions. However, the trend of reduction was less observed regarding eye problems. Ocular complications were described as idiosyncratic and may occur shortly after, or weeks, months, or even years after ZA treatment (33). Therefore, all patients who complained of eye symptoms were referred for ophthalmologic consultation. However, our findings regarding the reduction of occurrence of other adverse effects with multiple infusions among ZA-treated patients were in agreement with previous studies (25, 34). Therefore, patients undergoing ZA treatment should be informed about these symptoms and reassured that the incidence will decline with subsequent infusions. In addition, these symptoms should not affect the patient compliance toward ZA treatment and ZA re-dosing should be considered to obtain beneficial therapeutic effects even in those who have experienced symptoms (25).
The effect of ZA treatment on the serum levels of different biochemical parameters was also assessed as they could indicate the presence of underlying problems that might be asymptomatic or not reported by patients such as renal failure and hypocalcemia. However, according to the findings, ZA had no significant effect on investigated parameters. Patients at KAUH are usually prescribed prophylactic oral calcium and vitamin D before ZA treatment, which might give explanation for calcium levels being unchanged with ZA infusions. Furthermore, it might explain why the phosphorus level is also unchanged since the mechanism of ZA induced hypophosphatemia has been suggested to be caused by secondary hyperparathyroidism as a result of the hypocalcemia (35). The results of the present study suggested that the absence of asymptomatic or unreported adverse effects such as renal failure and hypocalcemia. These findings support previous studies and suggest that ZA is a safe modality in the treatment of SRE among patients with cancer (3, 23, 24).
Observations from this retrospective, single-center study need to be confirmed by studying a larger cohort of patients, ideally in prospective studies from multiple hospitals. Other limitations might include heterogeneity in gender distribution, cancer type, and indications for ZA treatment.
5.1. Conclusions
Within the limitations of this study, the findings demonstrated that ZA treatment in patients with cancer was not associated with major adverse effects even with multiple infusions. ZA acute events such as myalgia, bone pain, influenza-like symptoms, headache, and pyrexia were self-limiting and reduced with repeated infusions. In addition, ZA had no significant effect on the serum levels of biochemical parameters linked to medication toxicities. Stringent pre-infusion screening of co-morbidities is highly recommended to reduce the incidence of serious adverse events such as renal failure, atrial fibrillation, and jaw osteonecrosis.