1. Background
Radiotherapy is a well-known modality of cancer treatment artillery. In order to obtain optimal results in radiotherapy, an appropriate balance must be established between the fraction size, the total radiation dose, and the natural tissue threshold. In fact, the effects of radiation on the natural cells and adjacent tissues of the tumor limit the use of higher doses in radiotherapy. Ionizing radiation, as low Linear Energy Transfer (LET) as x-rays or γ- rays, creates free radicals in the cell. These radicals are highly reactive and react with cellular macromolecules such as Deoxyribonucleic acid (DNA), Ribonucleic acid (RNA), and proteins, and will lead to disruptions and cell death. Radiation moderator agents that can specifically protect normal cells, but not cancer cells against radiation, or specifically increase the sensitivity of cancer cells to radiation, would improve the efficacy of radiotherapy (1).
In recent decades, in order to find suitable radio-protector and radio-synthesizer agents, wide studies have been done and various factors have been introduced. Among them, antioxidants that protect the cells against free radicals and oxidative stress are highly regarded. There is a hypothesis that suggests that high doses of dietary antioxidants (vitamins C and E, and β-carotene) may increase tumor response to radiotherapy and decrease the toxicity on normal cells (2).
Among the herbs containing antioxidants, saffron can be mentioned. Commercial saffron is produced from dried stigmas of Crocus sativus L., a member of the large family Iridaceae, widely cultivated in Iran. The history of saffron cultivation dates back to more than 3,000 years. The saffron stigma has a distinct and unique colour, flavour, and aroma and is used as a spice and food coloring. Saffron has been used in traditional medicines for the treatment of a wide range of sicknesses. Saffron contains an impressive variety of herbal ingredients that act as antioxidants such as crocin, crocetin, and safranal. Water-soluble carotenoids crocin and free-agent crocetin are saffron-coloured compounds; Picrocrocin is responsible for the bitter taste in saffron; the volatile oil safranal is responsible for saffron odor and aroma of saffron. Furthermore, saffron contains the pigments like anthocyanin, α-carotene, β-carotene, and zeaxanthin (3, 4). These components have various pharmacological effects including anti-tumor effects (4). It has been suggested that crocin is the main anti-tumor ingredient in saffron (5-7). Safranal also showed an inhibitory effect on some cell lines growth (8, 9). Aung et al. suggested that saffron extract and its main component, crocin, inhibit the growth of colorectal cancer cells without affecting normal cells (5).
According to the World Cancer Report (GLOBOCAN 2018), Colorectal cancer (CRC) is the second most common cancer in women and the third most common cancer in men and its incidence increases with age. Surgery, radiation therapy, and chemotherapy are among the main tools of colorectal cancer therapy. Efforts to improve the efficacy of radiotherapy in this cancer are continued. Theoretically use of radiation-sensitizing and radiation-protective agents can help to improve the outcome of cancer and reduce its mortality.
2. Objectives
In this study cytotoxicity and radiosensitivity of 3 saffron extracts were investigated on the human colorectal cancer and normal fibroblast cells. As these components have different polarities, we used polar, semi-polar, and non-polar extractions of saffron for more investigation about the effects of different extractions of saffron on cell radiosensitivity.
3. Methods
3.1. Preparation of the Saffron Extracts
Stigmata of saffron from Faizabad (a city in the northeast of Iran) were used in this study. At the first step for total extract (Polar extract) preparation, 18 g of dried stigma was soaked in 70mL of methanol 96% at 4°C for 72 hours. The prepared extract was concentrated to 100 mL with a rotary evaporator in low pressure. For semi-polar and non-polar isolation (extraction and separation) 100 mL (in 5 steps) of dichloromethane or ether de petrol was added to 50 mL total extract in a separator funnel, respectively. Solvents were removed from samples by evaporation using a rotary evaporator and were stored at -20°C.
Various concentrations of saffron extracts (50, 100, 200, 400, 500, and 750μg/mL) and the control solutions without saffron extracts were prepared in Roswell Park Memorial Institute (RPMI) cell culture medium (for polar extract) and Dimethyl sulfoxide (DMSO) solvent (for semi-polar and non-polar extracts). These agents were refrigerated before the experiments.
3.2. Cell lines and Cell Cultures
Human colorectal cancer cells (HT-29) and normal human fibroblasts were purchased from Pasteur, Iran. The cells were grown in RPMI-1640 culture medium supplemented with 10% fetal bovine serum and penicillin/streptomycin at 37°C with 5% CO2 and saturated humidity. The cells were maintained in an exponential growth phase by changing the medium every 2–3 days. When the cells reached about 80% confluence, they were trypsinized, harvested, and seeded into a new tissue culture flask. Cell survival rates were determined by MTT assay. For this purpose, the cells were seeded in 96 well plates and were allowed to adhere for 24 h, and then were subjected to the designed experiments.
3.3. Saffron Extracts Cytotoxicity Assay
Human colorectal cancer cells (HT-29) and human fibroblast Cells were seeded in clear 96-well plates at a density of 5000 cells/well. After 24 h, different saffron extracts were added at final concentrations of 100, 200, 400, 500, and 750 μg/mL. Control solutions were considered as blanks. Cells were incubated at 37°C for 24 h in a 5% CO2 atmosphere. Cell numbers were evaluated using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. To each well, 20μL MTT (5mg per mL in Phosphate-buffered saline (PBS)) was added and the medium was removed after 4 hours. The formazan crystals were dissolved in 100μL DMSO and the absorption was measured at 570nm in an enzyme-linked immunosorbent assay (ELISA) reader. Assays were carried out in triplicate. Furthermore, as the control groups of radiosensitivity assay, after saffron extracts elimination cells were incubated for more 72h and then MTT assay was carried as described.
3.4. Radiosensitivity Assay
Cells were seeded in two 96-well plates (5000 cells/well). After 24 h, 100 μL of various concentrations of different extracts (50-750 μg/mL) and control solutions were added to the wells as mentioned. The medium was replaced with 100 μl of the fresh medium after 24 h of incubation. One plate received a single dose of 8 gray (Gy) of X-rays and one as the control group did not receive any radiation. Cell growth was evaluated using MTT assay as described after 72h re-incubation. All experiments were performed at least in triplicate.
3.5. Statistical Analysis
One-way ANOVA and Tukey post-hoc tests were used to assess the significant differences between the various groups. P values < 0.05 were considered statistically significant. Data are presented as mean ± standard error (SE).
4. Results
4.1. Saffron Extracts Cytotoxicity
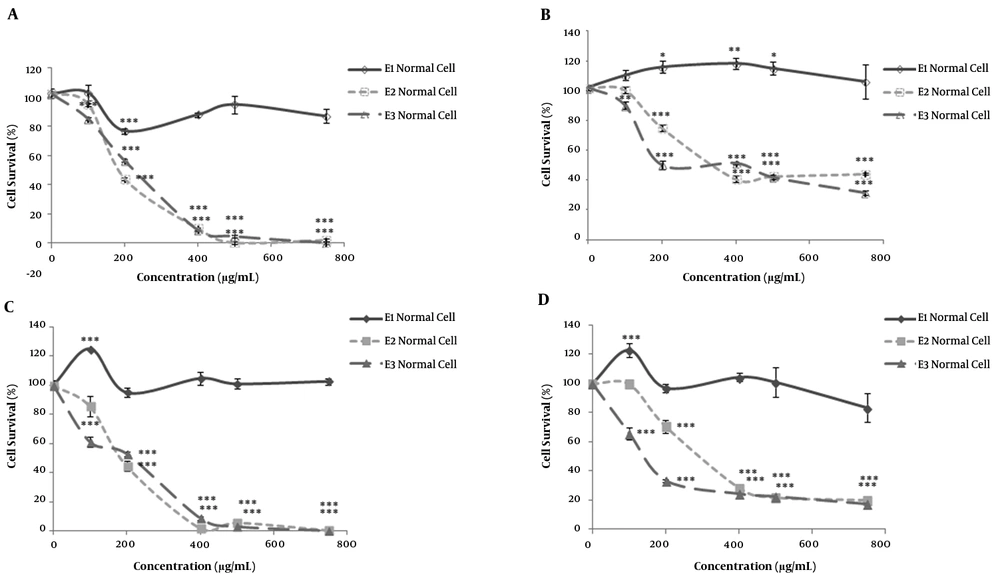
Human colorectal cancer cells (HT-29) and human fibroblast Cells (as non-malignant control cells) were separately incubated with various concentrations (100–750 μg/mL) of polar (E1), semi-polar (E2) and non-polar (E3) saffron extracts for 24 h. The results of immediate MTT assay showed a reduction in cell viability for semi-polar (E2), and non-polar (E3) saffron extracts in both cell lines, as a concentration-dependent manner (Figure 1A, 1B). While polar extract (E1) only in normal cells reduced cell viability at the concentration of 200 μg/mL significantly (P < 0.001) (Figure 1A). E1 in tumor cells not only failed to show any significant cytotoxicity but could also induce significant cell growth in different concentrations (Figure 1B). In the radiosensitivity control group, the cell growth in low dose concentration of 100 μg/mL significantly increased in both cell lines after more than 72h cell incubation (Figure 1C, 1D).
Doses inducing 50% growth inhibition (IC50) in the normal and tumor cells are presented in Table 1. These data demonstrated the cytotoxicity effects of the non-polar extract on tumor cells are more than the semi-polar extract while these two saffron extracts had almost similar cytotoxicity in normal cells. Furthermore, the data of these experiments demonstrated more cytotoxicity at the different saffron extracts in the normal cells relative to the tumor cells.
The cytotoxicity effects of different polar saffron extracts evaluated by MTT assay. Two groups of human colorectal cancer (HT-29) and normal fibroblast cells incubated with polar (methanolic, E1), semi-polar (dichloromethane, E2), and non-polar (ether de petrolic, E3) saffron extracts at different concentrations (100 -750 μg/mL) for 24h. MTT assay carried out for one group immediately (A, B), and the culture medium for the other group replaced with a fresh medium and incubated for more than 72h (C, D). Means ± SEM are shown (n= 3; *P < 0.05, **P < 0.01, ***P < 0.001). Cell survival in untreated cells was taken as control (100%).
| Saffron Extract | MTT assay 24h | MTT assay after more than 72h | ||
|---|---|---|---|---|
| IC50 Normal Cells | IC50 Tumor cells | IC50 Normal Cells | IC50 Tumor cells | |
| Polar (E1) | > 750 μg/mL | > 750 μg/mL | > 750 μg/mL | > 750 μg/mL |
| Semi-Polar (E2) | 201 μg/mL | 418 μg/mL | 190 μg/mL | 302 μg/mL |
| Non-Polar (E3) | 202 μg/mL | 304 μg/mL | 172μg/mL | 176 μg/mL |
4.2. Cell Radiosensitivity
For investigation about the radiosensitizing effects of different polar extracts of saffron on normal and tumor cells, we had two similar extract-treated plates for each cell line. One received 8 Gy of X-rays and the other one used as the control group. After 72 h incubation, cell growth was evaluated using MTT assay as described. For radiosensitizing effects description we defined 2 factors including relative cell death and relative synergism that were calculated using the following equations:
In the cases that the extract did not cause any cell death, cell death considered 0 in calculations. The ideal condition for one extract to be recognized as a radiosensitizer drug is that both of these 2 factors be > 1 in tumor cells whereas be ≤ 1 in normal cells. The results are illustrated in Figures 2 and 3 and significant findings have been marked.
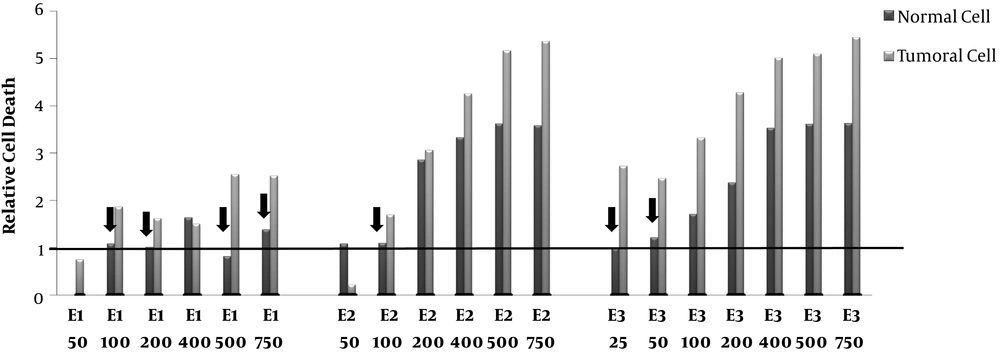
Relative Cell death. Human Colorectal Cancer (HT-29) and normal fibroblast cell lines incubated with various concentrations (50-750 μg/mL) of polar (methanolic,E1), semi-polar (dichloromethane, E2), and non- polar (ether de petrolic, E3) saffron extracts for 24h.The cells received 8 Gy of X-rays after more than 72 h incubation, cell growth was evaluated using MTT assay. The ratio of cell death induced by combination therapy to cell death induced by single radiotherapy calculated as relative cell death. The arrow signs point to the cases that relative cell death is >1 in tumor cells whereas ≤ 1 in normal cells.
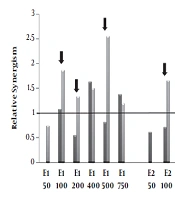
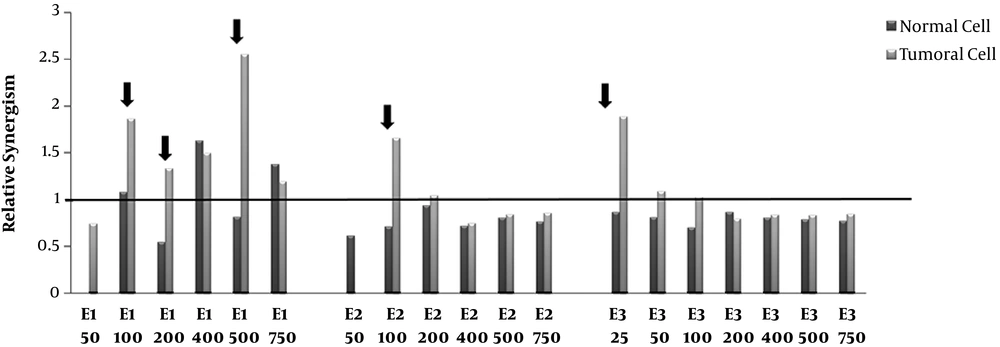
Relative synergism. Human Colorectal Cancer (HT-29) and normal fibroblast cell lines incubated with various concentrations (50-750μg/mL) of polar (methanolic, E1), semi-polar (dichloromethane, E2) and non- polar (ether de petrolic, E3) saffron extracts for 24h. Then, one plate received 8 Gy of X-rays and one as a control group did not receive any X-ray radiation. After more 72 h incubation, cell growth was evaluated by MTT assay. The ratio of cell death induced by combination therapy to the sum total of cell death induced by the single extract and single radiotherapy calculated as relative synergism. The arrow signs point to the cases that relative synergism is > 1 in tumor cells whereas ≤ 1 in normal cells.
4.3. Relative Cell Death
The effects of combination therapy of different concentrations of saffron extracts and radiotherapy (8GY) on normal and tumor cells compared to the cells which did not receive any extract are displayed in Figure 2. The results demonstrated that the combination therapy of polar saffron extract (E1 in the doses (100, 200, 500, 750μg/mL)) could increase cell death in tumor cells about (1.6-2.5)-fold. E1 was unable to increase the radiosensitivity of tumor cells in a low dose (50μg/mL). On the other hand, polar extract (E1) did not have any radiosensitizing effects on normal cells at 100 and 200 μg/mL concentrations and furthermore could protect normal cells with a concentration of 500 μg/mL.
In the cases of semi-polar (E2) and non-polar (E3) extracts, although tumor cells death increased up to 6 -fold in high doses (≥ 200) but also cell death increasing in normal cells has been shown. These high doses of the extracts cannot be suitable for radiosensitizing. In contrast, intermediate dose of E2 (100 μg/mL) and low doses of E3 (25 and 50 μg/mL) increased relative cell death just in tumor cells (1.7, 2.7 and 2.5-folds, respectively) and can be considerate as a good candidate for radiosensitizing. Since just non-polar extract (E3) in a low dose (50 μg/mL) could increase relative cell death in tumor cells, we also examined the lower dose of E3 (25 μg/mL).
4.4. Relative Synergism
The relative synergism factor indicates the combination therapy of different polar saffron extracts and X-ray, which significantly increases the rate of cell death compared to the total of individual induced-cell deaths due to the extract and radiotherapy.
According to Figure 3, combination therapy with polar saffron extract (E1) at all mentioned doses except 50 μg/mL showed synergistic effects in increasing cell death in tumor cells (about 1.2-2.5-fold) while it did not show any distinctive synergistic effect in normal cells at (100, 200, 500μg/mL) concentrations.
These are while semi-polar extract (E2) just at the concentration of 100 μg/mL and non-Polar extract (E3) at the concentration of 25 μg/mL showed 1.7 and 1.9- fold synergistic effects on tumor cells, respectively. These two extracts did not show any synergistic effects on normal cells.
5. Discussion
Natural medicinal resources have long been appreciated due to their potential effectiveness and low side effects. In the field of cancer treatment, the adverse effects of chemical radiation-protective agents made researchers interested in finding new plant-based radiation modifying agents. A radiation modifying agent must selectively enhance the effect of irradiation on tumor cells, but not on normal cells. Furthermore, it is better to selectively protect normal cells against radiation damage (10).
One of the major limitations of radiotherapy is tumor hypoxia. Oxygen by forming DNA-damaging free radicals is a potent radiation-sensitizing agent. Tumor cells in a hypoxia condition may be 2 or 3 times more resistant to radiation damage (11). Reactive oxygen species (ROS) by mediating oxidative stress and triggering apoptosis plays a key role in killing the tumor cells and radiosensitivity enhancement. The cellular radiosensitivity is linked to the level of ROS in the cell. Normally, ROS exist in high levels in tumor cells and so can be a target for selective killing of tumor cells (10). Furthermore, some studies proposed that antioxidants can be used as selective radiation modifying agents without any considerable toxicity in humans. It has been suggested that the combination of antioxidants and radiotherapy can enhance the radiosensitivity of tumor cells by increasing Ros production (2, 10). However, the mechanism of these contradictory effects of antioxidants on tumor and normal cells is still a conundrum. These compounds may produce biological effects on cancer cells by mechanisms that are not related to their antioxidant action (2).
The cytotoxic and anti-tumor activities of saffron extracts have been shown in several studies (3-9). Saffron purified carotenoids (crocin, crocetin) and monoterpene aldehydes (picrocrocin and safranal) show different degrees of cytotoxic and anti-tumor activities. Since saffron components like crocin, crocetin, safranal, and β-carotene showed antioxidant activities (12-15), we decided to investigate the potential of different saffron extracts for using as radiation-sensitizing agents. For this purpose, polar (methanolic, E1), semi-polar (dichloromethanic, E2), and non-polar (ether de petrolic, E3) extractions of saffron were prepared and their ability to use as radiation-sensitizing and radiation-protective gents were tested in human colorectal cancer cells (HT-29) and human normal fibroblast cells, respectively. Base on the solubility of the saffron compounds, crocin as the polar water-soluble carotenoid, was considered to be the major component of polar extract (total extract) while safranal and β-carotene were considered as the major components of the non-polar extract. The semi-polar extract was assumed to contain crocetin and has a lower percent of safranal and β-carotene compared with the non-polar extract.
At the first step, we assayed the growth inhibitory activity of methanolic extract as a polar saffron extract on human colorectal cancer cells (HT-29) and human fibroblast cells. We found that the concentrations inducing 50% cytotoxicity (IC50) of this extract on both cell lines were more than 750 μg/ml. We observed saffron polar extract, at the tested concentrations (up to 750 μg/ml), not only didn’t have any cytotoxic effects on tumor cells but also could increase cell survival in different doses. Polar extract in low concentrations has increased cell survival to some extent in normal cells too, although not significantly. This is while stimulating the growth of some cancer cells with low doses of antioxidants like vitamin C (16) and polar carotenoids (17) in culture mediums had been seen previously. Another study performed by Aung et al. had shown that saffron total extract reduces the proliferation of HT-29 cells at the concentration of 3 mg/ml significantly (4).
The comparison of different saffron polar extracts IC50 revealed that by reducing the polarity of saffron extracts, the cytotoxicity increases in both cell lines. These results should be because of crocin, crocetin, and safranal cytotoxicities. Escribano et al. demonstrated the non-polar component of safranal is the most toxic component of saffron on human cancer cells while the toxicity effects of crocetin and crocin reduce respectively (9).
On the other hand, our experiments indicated that the cytotoxicity effects of all saffron extracts on normal fibroblast cells were more than tumoral HT-29 cells. These results are inconsistent with some studies that revealed saffron is more toxic in tumor cells compared with normal cells. For example, Aung et al. demonstrated total saffron extract did not show any significant inhibition of the non-cancer cell of colon in young adult mouse (5). Tavakkol-Afshari et al. reported that ethanolic saffron extract is selectively cytotoxic against HepG-2 and HeLa cells but it is nontoxic on normal mouse fibroblast cells (L929) (18). However, because of the differences in cell types and experimental the conditions, comparison is not plausible.
According to the relative cell death and synergism index, it seems that the polar saffron extract at 100, 200, and 500 μg/mL concentrations, semi-polar saffron extract at 100 μg/mL concentration, and non-polar saffron extract at 25 and 50 μg/mL concentrations can act as radiation modifying agent in colorectal radiotherapy. These results demonstrated that a suitable dose to use as radiosensitizers decreases by saffron extracts polarity reduction. At higher doses of all extracts, no synergistic effect was observed due to high toxicity. Therefore, it seems antioxidants in lower toxic doses can increase the radiosensitivity of tumor cells without any effects on normal cells. The results of the present study confirm the hypothesis of Prasad et al. (2) about antioxidant use during radiation therapy to some extent. This hypothesis states radiotherapy in combination with high doses (not toxic dose) of dietary antioxidants like vitamins C and E, and carotenoids can increase tumor cells response without any adverse effect or still with protective effects on normal cells.
5.1. Conclusion
Concurrent use of polar saffron extracts and a low dose of non-polar saffron extracts and radiation can enhance radiation sensitivity and cell death in tumor cells, while might be expected a normal tissue sparing effect. Therefore, we suggest these saffron extracts to be considered as a potential co-administration drug and radiation-sensitizing agent in cancer treatment for further studies.