1. Background
Proton therapy has been used in hospitals in the last 2 decades as a relatively new treatment modality for cancer. The first suggestion for using the energetic protons in radiotherapy was made by Robert R. Wilson in 1946 (1). As a radiotherapy method, proton therapy has numerous radiobiological and physical advantages over other radiotherapy methods. All radiotherapy methods aim at destroying tumor cells and at the same time minimize inadvertent damage to adjacent cells. Schardt et al. (2010) and Loeffler and Durante (2013) showed that in ion radiotherapy, the dose can be deposited over a small range and, therefore, damage to healthy tissues is reduced considerably (2, 3). Proton-beam re-irradiation therapy is a safe and effective curative strategy, with an acceptable rate of toxicity and durable disease control (4). In recent years, the use of high-Z nanoparticles, as radio-sensitize agent, has been proposed as a breakthrough in radiotherapy (5). Various experiments (6-9) and numerical simulations (10, 11) have been carried out to study radio-sensitization effects of using gold nanoparticles (GNPs) in photon radiation fields. Studies on the effects of metal -nanoparticles, such as gold, silver, platinum, and gadolinium, in combination with ionizing radiation, have revealed that the sensitization by nanoparticles enhances the effects of radiation (12-15). Due to their high atomic number, biocompatibility and potential for targeted surface modification, GNPs have attracted a lot of attention (16). The composition that comprises nanoparticle has bigger molecules than the nutrients in the vessel. Also, cancer cells are more active and capillaries in tumor area are bigger compared to the healthy tissues. Therefore, most of nanoparticle compositions are absorbed in the tumor area. Some secondary particles are created through the nuclear interactions between the beam and nanoparticles, resulting in an increase in dose in the area containing nanoparticles. GNPs can be bound to many proteins and drugs and can be actively targeted to cancer cells overexpressing cell surface receptors (17). Likewise, protons have a high cross-section with gold at a wide range of relevant clinical energies, and as a result, they can be potentially used with GNPs for increased therapeutic effect (18).
Kim et al. (19) have stressed the role of secondary electrons and the characteristic X-rays emitted from metallic nanoparticles irradiated by protons and observed complete tumor regression as well as increase in intracellular reactive species level in mice tumors. Hainfeld et al. used gold particles with a diameter of 1.9 nm to demonstrate the increased effect of radiotherapy on mice. They found out that during radiotherapy, the ratio of gold density in tumor tissue compared to the healthy tissue remains 8 to 1 (6, 7). In the research by Christopher et al., a considerable increase in the living cells of the prostate tumor has been reported when sensitized by GNPs and exposed to 160MeV proton beams (20). Since the safety tests regarding the clinical use of nanoparticles have been conducted, some computational studies on the important factors in this method should be carried out. The studies conducted on this therapeutic method for the tumor sensitization by GNPs have been mostly qualitative, while there are only a few quantitative studies on dose enhancement and factors affecting it. Martinez-Rovira and Y. Prezado studied the local dose enhancement in combination of proton therapy and nanoparticles by Monte Carlo (MC) simulation (5). MC’s method is a robust method for the simulation of the particle transport. In this method, a statistical method, like the one occurring in reality, is developed and, then, repeated several times with the aid of random numbers and random occurrence of the phenomenon in question. Therefore, the simulations with MC method can be considered a theoretical experiment. Their work was divided into 2 steps, including a macroscopic simulation of a proton beam impinging on a water phantom and a nanometric simulation to assess dose distributions around the nanoparticles. Walzlein et al. (8) made MC simulations, using the track structure code TRAX to investigate a possible dose enhancement effect by proton or electron irradiation in the vicinity of nanoparticles consisting of different high Z atomic materials. Tran et al. (21) presented an in silico investigation, on the basis of the general purpose MC simulation toolkit Geant4, into energy deposition and radical species production around a spherical GNPs 50 nm in diameter via proton irradiation.
The aim of this article is to employ MC simulation to examine dose enhancement effect in proton therapy by implementation of exact tumor compositions of different GNP densities. When the protons pass through the matter, they interact with the atoms, and secondary particles such as neutrons and photons are produced. These particles may scatter into other parts of the body and their dose may cause secondary cancer. Therefore, the objective of this paper is to study both primary and secondary particles with and without GNPs radio-sensitization. Below, SOBP, photon, and neutron spectrum, as secondary particles for 2 situations, have been studied.
2. Methods
Simulation for calculation of flux and energy deposition was conducted, using MCNPX code, version 2.4. In simulations of proton therapy, MC methods usually use a combination of continuous processes based on condensed history and discrete processes, which are nuclear interactions, secondary particle production, and Coulomb scattering, based on an explicit model of each interaction (22) For maximum precision, the energy considered here was an equivalent of the least amount of energy that can be traced by MCNPX code, i.e. 1 KeV for photon and electron and 1 MeV for neutron and proton. A number of 200000 protons were traced to achieve a relative error of less than 0.01.
2.1. Head Phantom
The overall geometry for the head phantom used in this study is a layered cube with these specifications: 0.2 cm human skin, 0.3 cm soft tissue, 0.9 cm skull bone, and 11.5 cm brain, 0.9 cm skull bone, and, finally, 0.5 cm soft tissue. The lateral dimensions of the phantom were considered in accordance with the maximum values of brain in the MIRD-ORNL phantom as 17.2 cm × 13.2 cm (23).
Type and elemental composition of the tissues in the head phantom are expressed as weight percentage in Table 1 (24). Tumor specifications with the homogenized GNPs agents with different concentrations of 10, 25, 50, and 75 mg GNPs per mL of tumor material are presented in Table 1. Data on gold concentration, as homogenous distributions, were extracted from technical texts (25-27).
| Tissue Type | Density (g/cm3) | H | C | N | O | Ca | Na | P | S | Cl | K | Au |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin | 1.09 | 10.0 | 20.4 | 4.2 | 64.5 | - | 0.2 | 0.1 | 0.2 | 0.3 | 0.1 | - |
| Soft tissue | 1.03 | 10.5 | 25.6 | 2.7 | 60.2 | - | 0.1 | 0.2 | 0.3 | 0.2 | 0.2 | - |
| Skull bone | 1.61 | 5.0 | 21.2 | 4.0 | 43.5 | 17.6 | 0.1 | 8.1 | 0.3 | - | - | - |
| Brain | 1.04 | 10.7 | 14.5 | 2.2 | 71.2 | - | 0.2 | 0.4 | 0.2 | 0.3 | 0.3 | - |
| Non-sensitized tumor | 1.04 | 10.7 | 14.5 | 2.2 | 71.2 | - | 0.2 | 0.4 | 0.2 | 0.3 | 0.3 | - |
| Sensitized tumor containing different concentration of GNPs | ||||||||||||
| 10 mg Au mL-1 | 1.05 | 10.6 | 14.4 | 2.2 | 70.5 | - | 0.2 | 0.4 | 0.2 | 0.3 | 0.3 | 1.0 |
| 25 mg Au mL-1 | 1.07 | 10.4 | 14.2 | 2.1 | 69.5 | - | 0.2 | 0.4 | 0.2 | 0.3 | 0.3 | 2.3 |
| 50 mg Au mL-1 | 1.09 | 10.2 | 13.8 | 2.1 | 67.9 | - | 0.2 | 0.4 | 0.2 | 0.3 | 0.3 | 4.6 |
| 75 mg NP mL-1 | 1.12 | 10.0 | 13.5 | 2.1 | 66.4 | - | 0.2 | 0.4 | 0.2 | 0.3 | 0.3 | 6.7 |
Mass Density and Elemental Composition of the Tissues in the Head Phantom for Sensitized and Non-Sensitized Tumora
2.2. Beam Configuration
In Hadron therapy with modulated intensity, pencil beam controlled protons are used, in which the intensity of pencil beams can be controlled at a small level to precisely target a tumor. Pencil beam scanning system, also known as spot scanning system, substantially reduces the secondary particle production. Here, we only concentrate on the interaction between proton beam and body tissue. A pencil proton beam with a radius of 0.01 cm is vertically irradiated on the surface of the phantom. The beam is considered to have 7 mm of FWHM at the skin surface (28).
3. Results
3.1. Pristine Bragg Peak and SOBP
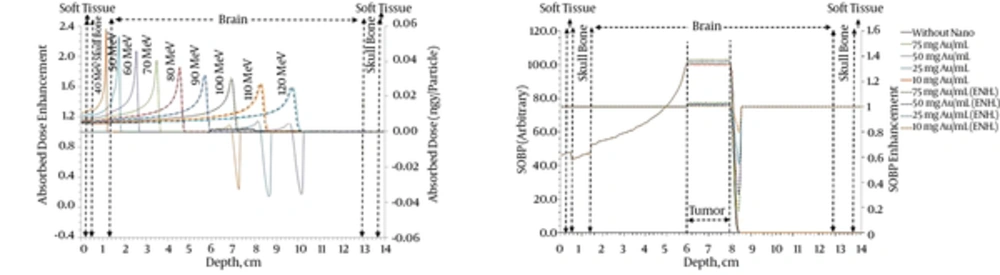
Depth dose profile was calculated for different levels of energy, including; 40, 50, 60, 90, 100, 110, 120, 130, 140, and 150 MeV. To determine the energy dependency of the enhancement of pristine depth dose, a 2 cm tumor within the depth of 6 and 8 cm of the brain with 75 mgAu/mL and without it is considered. The obtained dose profile for 2 cases of the tumor without GNPs and radio-sensitized tumor with 75 mgAu/mL are presented in Figure 1A. To evaluate the effect of GNPs, the dose enhancement factor, which is defined as the ratio of the dose sensitized by GNPs in the tumor to the dose in the normal tumor, is calculated. Figure 1A shows the dose enhancement resulting from the presence of a 75 mgAu/mL compared to a state where there are no GNPs. It can be seen that the power of sensitization by GNPs for energies with Bragg peak in the tumor area is significant. It can be observed that all beams with energies higher than the energies in the tumor area show a steady increase, which is clearly because of the GNPs; and the value-shaped decrease after the Bragg peak is due to the significant effect of GNPs.
The deposited energy in the medium increases by decreasing proton velocity and comes to a pick when proton comes to rest (Bragg pick). If the thickness of the tumor is more than the size of the Bragg peak, radiation with a single Bragg peak would not be enough. Therefore, by combining several corresponding Bragg peaks with different beam energies, they will be as broad as the treated volume and, thus, the concept of SOBP is created. The SOBP is the sum of several individual Bragg peaks at staggered depths. Using the single depth dose profile resulting from simulations for beams with different energies, both in the presence and absence of gold particle sensitizers, the weight of each single peak is calculated to produce SOBP and distribute homogenous dose in the depth of the target. To calculate the enhancement of SOBP in the tumor in the presence of GNPs sensitizers, the same weights, as before, are used. Thus, first, a 2 cm tumor was considered to be located in a depth corresponding to 100 MeV protons. The beam displacement of 1.2 mm, equal to the average standard deviation of the 100 MeV proton Bragg peak, is considered to have a nearly uniform dose distribution without ripples in the tumor region (28). Therefore, by 18 depth dose profiles corresponding to 18 energies in the range of 91.6 to 108.2 MeV, and by considering the proper weights for each profile a flat SOBP without triples in the tumor region was obtained. Weighted pristine depth dose profiles and dose enhancement for GNPs embedded homogeneously in the described tumor and irradiated with a proton beam are shown in Figure 1B.
Figure 1B depicts a flat dose enhancement within the tumor and a valley-shaped dose reduction immediately after that.
Based on Table 2, percentage dose enhancements are 0.4, 1.1, 2.0, and 3.0 % for GNPs aided tumor, and the maximum percentage reduced dose are 19.9, 45.3, 69.2, and 82.3% for the surrounding region. This result is an important result from the viewpoint of the radiation treatment goal, i.e. “minimizing damage to the surrounding healthy cells” while “increasing damage to tumor cells”.
| No. | Cases | SOBP | Percentage Enhancement | Maximum Percentage Dose Reduction After Tumor |
|---|---|---|---|---|
| 1 | Non-sensitized tumor | 100.0 | - | - |
| 2 | 10 mg Au mL-1 | 100.4 | 0.4 | 19.9 |
| 3 | 25 mg Au mL-1 | 101.1 | 1.1 | 45.3 |
| 4 | 50 mg Au mL-1 | 102.0 | 2.0 | 69.2 |
| 5 | 75 mg NP mL-1 | 103.0 | 3.0 | 82.3 |
SOBP and Its Enhancement for Different Concentrations of GNPs
3.2. Secondary Particles Production
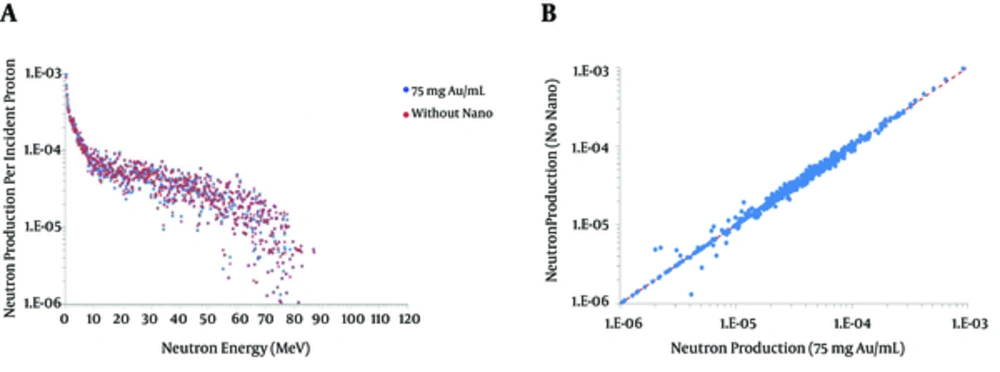
Production of secondary particles due to the nuclear interactions in the brain material through the beam line is one of the major concerns of proton therapy. Neutron spectrum shows a little deviation in the case of the radio-sensitized tumor with 75 mg Au/mL. Figure 2 do not reveal any absolute reduction or increase in neutron production; however, the average percentage enhancement of 0.4, 0.9, 1.4, and 1.7 % corresponding to 10, 25, 50, and 75 mg Au/mL, is presented in Table 3.
| No. | Cases | Average Neutron Production | Percentage Enhancement |
|---|---|---|---|
| 1 | Non-sensitized Tumor | 3.68e-2 | - |
| 2 | 10 mg Au mL-1 | 3.69e-2 | 0.4 |
| 3 | 25 mg Au mL-1 | 3.71e-2 | 0.9 |
| 4 | 50 mg Au mL-1 | 3.73e-2 | 1.4 |
| 5 | 75 mg NP mL-1 | 3.74e-2 | 1.7 |
Average Neutron Production and Its Enhancement
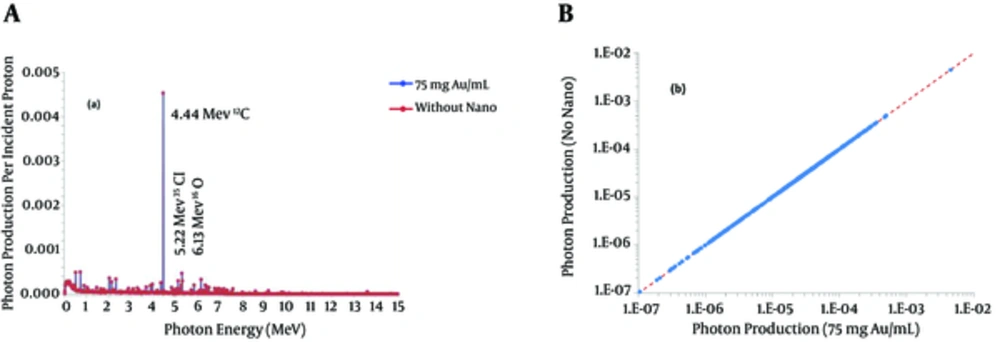
Figure 3A shows the energy spectrum of the photon produced by 100 MeV incident protons. Figure 3B outlines the comparison between photon produced by 100 MeV proton beam in non-sensitized and 75 mg Au/mL aided tumor. It is clear that the densities of GNPs studied here do not affect the production of photons significantly.
4. Discussion
4.1. The Impact of GNPs on Primitive Dose Profile
Tran et al. simulated a spherical gold nanoparticle of 50 nm diameter in an environment of water and irradiated by protons with energies between 2 and 170 MeV (21). Based on the results of their research, nanoparticle improves the dose in that area. According to a study carried out by Martinez and Prezado, larger dimensions were simulated (29). They concluded that by increasing the size of the study, the dose enhancement is reduced. Their research suggests more precise calculations based on a more realistic condition.
4.2. The Effect of GNPs on Real Tumor Geometry
In this study, exact tumor compositions of different GNP densities are implemented. SOBP and secondary particles production are studied for GNPs aided tumor. Several GNPs concentrations, including 10, 25, 50, and 75 mgAu/mL, are modeled homogeneously in a 2 cm tumor with the center located at a depth corresponding to 100 MeV proton pencil beam. Regarding the comprehensive studies performed on the enhancement of these parameters, it could be concluded that GNPs radio-sensitization can enhance the dose in Bragg peak. SOBP for radio-sensitization tumor is calculated based on weights obtained in the case of not aided tumor. This methodology reveals a flat dose enhancement in the tumor region and a dose reduction immediately after that. A dose reduction of up to 82.3% after the Bragg peak is a favorable result. A minor deviation appears for neutron production; however, photon production in all cases is almost the same for the 2 situations.
4.3. Conclusion
Previous studies on the effect of radio-sensitization on dose enhancement have been carried out by simulation of a simple and small dimension water environment. More realistic evaluation of dose enhancement in Nano-aided tumors using proton therapy, requires simulation of more realistic environment which is similar to a human head considering the compositions and dimensions. Therefore, this research study is conducted to simulate a real head phantom using the Monte Carlo approach. A 2 cm aided-tumor is modeled in a depth corresponding to 100 MeV proton pencil beam. Several concentrations of GNPs, including 10, 25, 50 and 75 mgAu/mL, are simulated to evaluate dose enhancement in Bragg peak, enhancement of SOBP and secondary particle production. A flat dose enhancement in the tumor region and a dose reduction up to 82.3% immediately after the tumor is a favorable conclusion. These results demonstrate the benefits of radio-sensitization method employment to improve proton therapy efficiency. Finally, it is concluded that neutron productions is affected by presence of GNPs, however, no change in photon production is obtained.