1. Introduction
Breast sarcomas are a group of heterogeneous tumors that constitute less than 1% of all primary breast cancers and less than 5% of all soft tissue sarcomas (1). Mammary stromal sarcoma (MSS) accounts for 0.03% of all primary breast malignancies among Iranian patients (2-4). It may contain heterogenous metaplastic components (e.g. muscle, bone, or cartilage) (5). A difficulty is considered in the management of MSS with chondroid differentiation, especially due to the undefined natural history. To our knowledge, no study has yielded the natural history of MSS with chondroid differentiation. This gap in the literature has hampered its management. In this case report, we try to give an example to shed light on the natural history of MSS with chondroid components.
2. Case Presentation
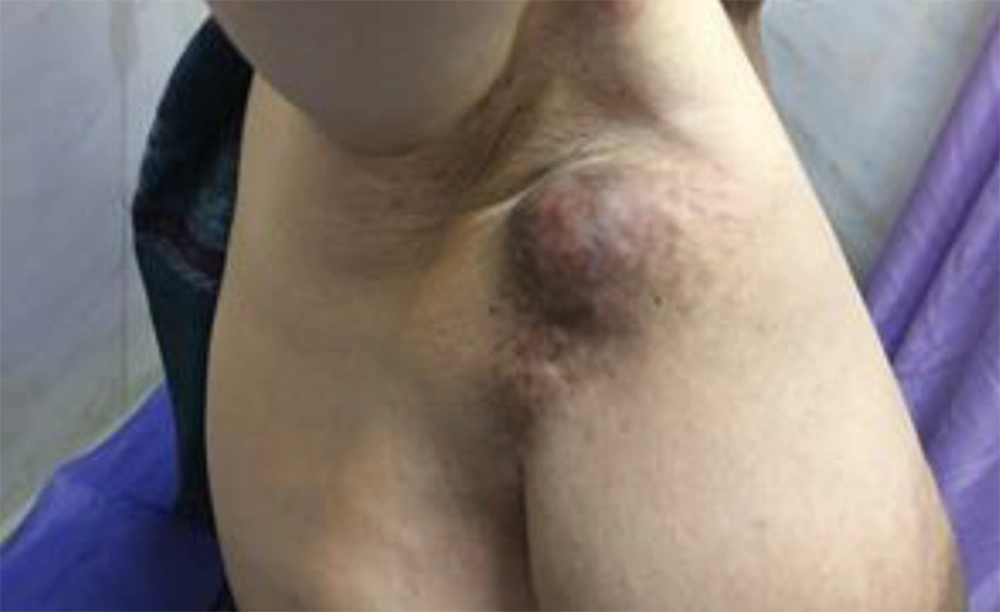
A 59-year-old female presented to our hospital with a chief complaint of armpit lump. Physical examination showed a large well-movable mass in her right axilla (Figure 1).
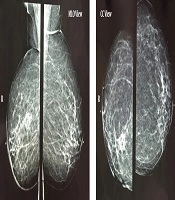
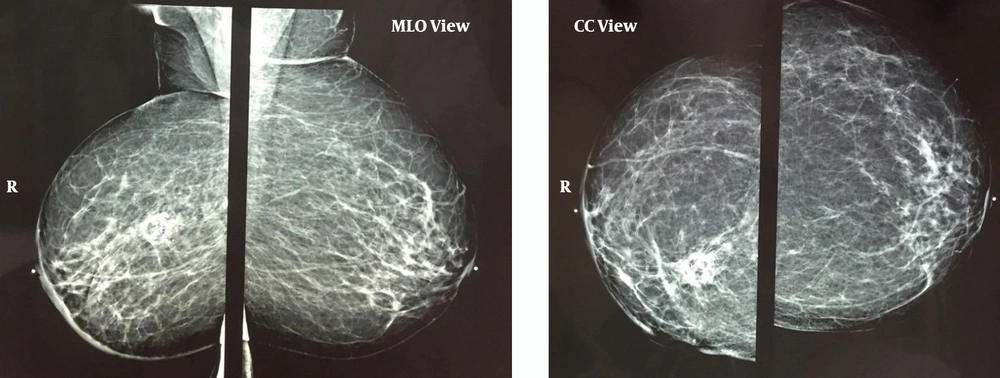
The physical examination of her breast was unremarkable. Her medical history was significant for right breast invasive ductal carcinoma, the status of post lumpectomy, chemotherapy, and radiotherapy of 5 years ago. Mammography demonstrated post-irradiation changes in the right breast (Figure 2).
Post-breast-conserving therapy scar is seen in the right breast, which contains central lucency and calcification due to fat necrosis. Diffuse Right breast skin and trabecular thickening accompanied by post-surgical distortion in the breast and axilla are visible. Axillary mass is not demonstrated in the mammogram. MLO, mediolateral-oblique; CC, cranial-caudal.
Based on ultrasonography (US), a lobulated heterogeneous hypoechoic soft tissue mass measuring 10 × 7 × 5.3 cm in the axillary tail of the right breast posterior to the pectoralis muscle was detected. She underwent US-guided fine-needle aspiration of the axillary mass. In the cytopathology report, no atypical cell was detected. Based on hematoxylin and eosin (H & E) stain of the core needle biopsy, the primary diagnosis was metaplastic breast carcinoma with chondroid differentiation. For confirmation of diagnosis, Immunohistochemical (IHC) staining was performed. The tumor tissue was positive for CD10 and vimentin, while it was negative for cytokeratin AE1/AE3, cytokeratin CAM 5.2, P63, CD34, epithelial membrane antigen (EMA), S100, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). The KI67 was positive in 5% of tumor cells. The final diagnosis was reported as CD10+ MSS with chondroid differentiation, possibly secondary to prior radiotherapy. No distant metastasis was detected in the initial staging workup. Because of the large tumor size, preoperative chemotherapy with doxorubicin plus ifosfamide (AI) regimen was planned. However, the tumor significantly progressed during chemotherapy courses, both local and systemic. Metastatic workup demonstrated 2 right-sided pulmonary nodules measuring 7 mm and 5 mm and mild right pleural effusion favoring metastatic involvement. The case was presented at the Tumor Board of Shohada-e Tajrish General Hospital. Due to the extension of the tumor, the surgical resection of the growing retro pectoral mass was unavailable. Considering the long interval from prior radiotherapy (RT), the palliative radiation of the chest wall mass was planned for her (6). Informed consent was taken from the patient.
3. Discussion
Radiation-induced sarcoma (RIS) is a well-known treatment complication that constitutes about 3% of all sarcomas (7). RIS was initially described in the early 1900s by Perthes et al. Later, the diagnostic criteria of RIS were introduced by Cahan et al. (8) as the lesions of different histology that are located at the RT field with a latency period of more than 4 years. In 2010, the sarcoma group of Memorial Sloan Kettering Cancer Center (MSKCC) modified this definition and declared that RIS may occur as soon as 6 months after RT (9). Over the past decades, the incidence of RIS has significantly risen (10). This may be due to an increase in the survival of irradiated patients by more effective systemic therapies, more utilization of RT (e.g. with the increased rate of breast-conserving surgery), and the increased use of modern RT techniques (e.g. intensity-modulated radiation therapy) (11). Studies have shown the poor prognosis of RIS in comparison with primary sarcomas. The RIS is relatively higher grade, larger, and deeper in location. Furthermore, prior RT impairs effective surgery and RT that further mitigates the prognosis (9). The MSS is a rare entity among Iranian females that accounts for 0.03% of breast cancers (2). It may be developed de novo or arise secondary to RT (12). In 1962, Berg et al. (13) defined the pathologic features of MSS. According to the 2013 World Health Organization classification of tumors, stromal sarcoma is categorized as the tumors with uncertain differentiation (14). Microscopically, MSS is characterized by spindle cells with nuclear atypia, brisk stromal mitotic activity, and IHC positivity for vimentin and CD10, and negativity for CD34 (15, 16). Histologically, its differential diagnosis is cystosarcoma phyllodes and metaplastic carcinoma. It can be differentiated from the former and later by sparing the lobular component and IHC characteristics, respectively (17). Because of the rarity of this disease, the therapeutic approach remains controversial. Similar to other sarcomas, surgery is the only potentially curative modality. Considering limited lymphatic involvement, wide local excision or mastectomy without lymphadenectomy is the most accepted surgical approach for MSS. The primary goal is resection of the tumor with negative margins (18). In the case of inoperable tumors, other modalities (e.g. chemotherapy or RT) can potentially facilitate the resection. However, the tumor of our case was resistant to the AI regimen. This case report can provide a clue to try alternative choices as neoadjuvant chemotherapy for MSS with chondroid components. Future studies can reveal this notion. Followings are learning points of this case report:
1) MSS is an extremely rare entity; so, the mainstay of therapy remains a major dilemma.
2) MSS has an aggressive natural history that intends to spread either locally or distant.
3) MSS with chondroid differentiation was resistant to the standard chemotherapy of sarcoma (i.e. AI regimen).
4) Radiotherapy is a potential choice in the case of chemoresistant MSS.