1. Background
Colorectal cancer (CRC) is a fatal and prevalent disease. According to the World Health Organization (WHO), there have been 4,789,635 CRC cases in 2018. CRC is the third most prevalent cancer and the third cause of cancer-related deaths in Iran (1). About 6% of CRC cases are related to hereditary factors and positive family history in terms of incidence and prevalence; so, it would be curable in the early stages of the disease (2). Lynch syndrome (LS), which is sometimes called hereditary non-polyposis colorectal cancer (HNPCC), is the most common inherited cancer disorder with an autosomal dominant inheritance caused by a germline mutation in one of the DNA mismatch repair (MMR) genes (MLH1, MSH2, MSH6 and PMS2) (3). Some studies have shown that the prevalence of HNPCC is between 2.0 and 10.7% of the total CRCs in Iran, demonstrating the high level of the LS prevalence (4-7). Since LS is one of the most important causes of CRC (8), it is important to determine the advisable diagnostic procedures for the recognition of LS patients (9).
In the past decade, the use of clinical and laboratory techniques and genetic testing for detecting hereditary syndromes has been common. For the detection of LS, some clinical criteria such as Amsterdam criteria (AC) II or revised bethesda guidelines, and some laboratory techniques including immunohistochemistry (IHC), microsatellite instability (MSI) testing, and next-generation sequencing (NGS) of MMR genes are currently used worldwide (10). The molecular testing techniques, such as IHC and MSI are performed on the tumor tissue specimens and their adjacent healthy tissues; they have a high power to screen the defective genes. Meanwhile, in genetic NGS testing, the blood DNA samples are usually used to detect the germ-line mutations in one of the MMR genes (11).
The molecular testing of the CRC patients at the risk of LS and their subsequent monitoring could lead to the early diagnosis of the disease, reducing the cost of the future treatment, ensuring longer life expectancy, and better quality of life in the individuals who are prone to this disease (12). However, molecular screening is often overlooked due to the costs. Many studies have been internationally conducted on the cost-effectiveness of the different approaches to screen LS. They have obtained favorable results in identifying LS and preventing CRC, thereby reducing the costs and improving the complications of the disease (9, 13-16).
A number of previous studies assessed the use of molecular tests such as IHC, MSI, NGS, and clinical criteria such as Bethesda guidelines and Amsterdam criteria. There are, however, some differences between the previous studies such as the way of setting up tests, the elimination of several tests, and the number of strategies considered and whether they are model-based or population-based (13, 17).
Although there are some studies on the molecular testing of CRC in Iran (18, 19), there has been no study on the cost-effectiveness of the molecular approaches in CRC. So, the present research is the first study estimating the incremental cost-effectiveness of molecular screening methods in the case of CRC patients; as well, there has been no screening that would incorporate quality-adjusted life years (QALY) and life-years gained in Iran. Since the molecular screening of CRC has not been widely considered in Iran so far, the results of this study can help decision-makers to identify the best screening strategies.
2. Methods
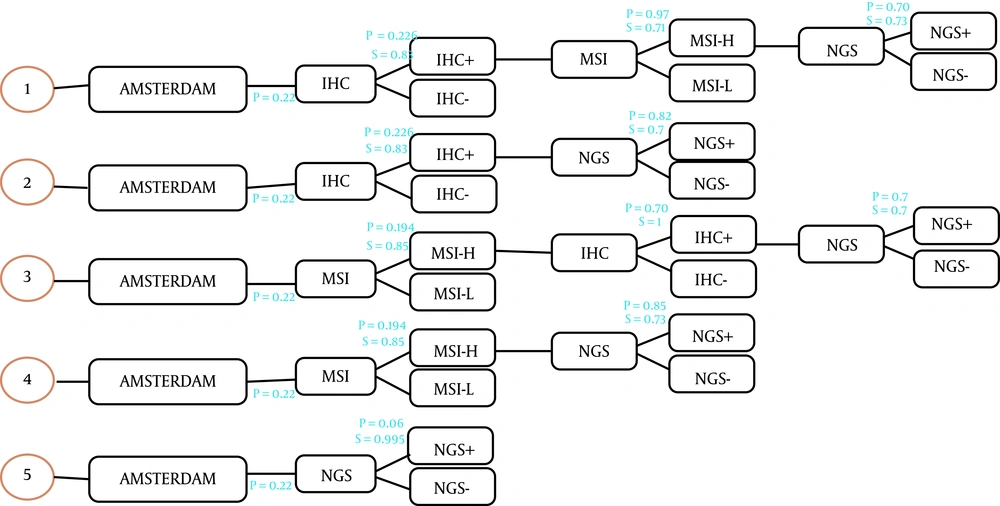
This study was a model-based economic evaluation. A decision tree model was accordingly designed to calculate the number of cases of LS detected in each strategy and the related costs. We evaluated the cost-effectiveness of different possible screening methods for diagnosing LS in a population with CRC. For this purpose, the costs and outcomes were calculated by adding colonoscopy care and aspirin intake in the form of surveillance programs for the relatives of the patients who carry LS. This study was conducted from the Iranian health care system perspective. The decision tree model started with 10,000 hypothetical patients with early-identified CRC and the number of identified LS cases was considered as clinically effective. The molecular methods used in this study are as follow: (1) IHC testing followed by MSI testing if the IHC result were defective. Then NGS if MSI were high; (2) IHC testing followed by NGS if the IHC result were defective; (3) MSI testing followed by IHC testing if MSI were high. Then NGS if IHC were defective; (4) MSI testing followed by NGS if MSI were high; (5) NGS. In all strategies, Amsterdam criteria II was considered the first step (Figure 1) and the comparator was no screening.
After identifying the CRC patients, if Amsterdam criteria were met, they would be offered a molecular test to detect Lynch syndrome. We assumed that all patients had agreed to participate in the tests. In the decision tree, the total cost and the number of the carriers of Lynch syndrome were calculated for each strategy, and only direct costs were considered. The sensitivity and specificity of the molecular tests were obtained from the relevant published literature (20, 21).
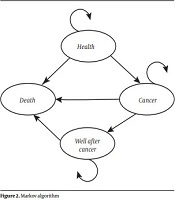
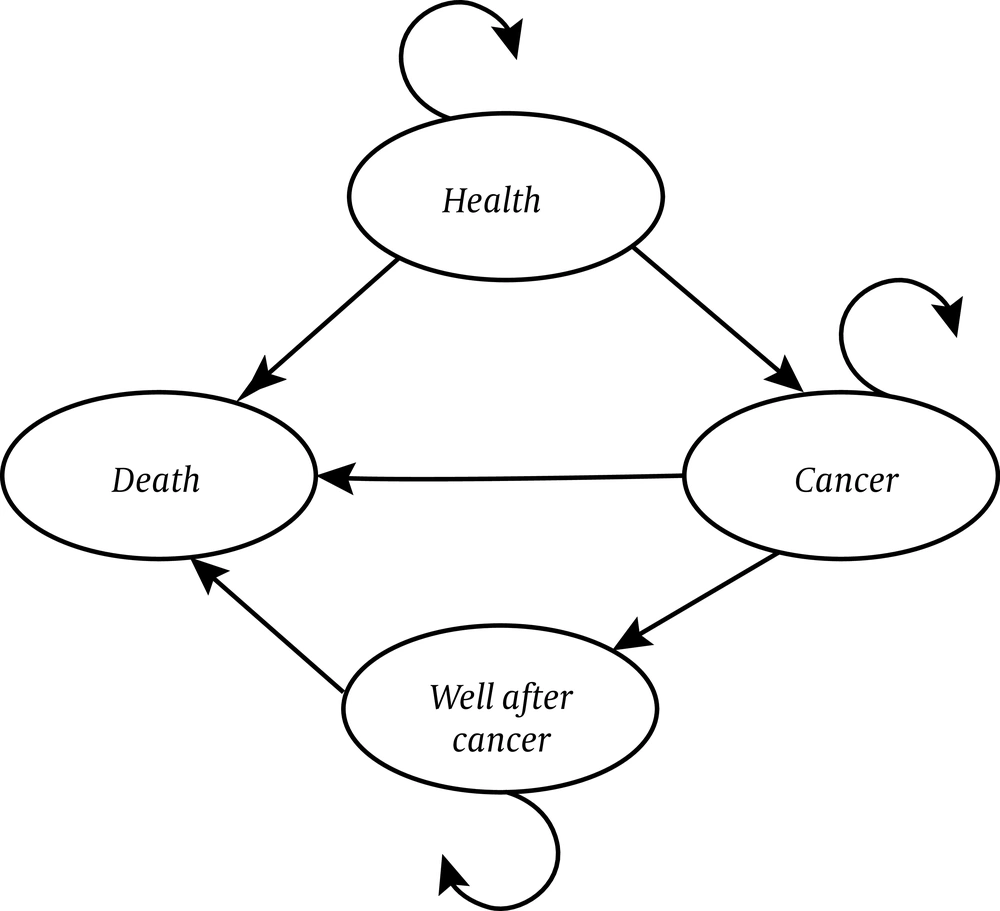
The Markov model was used to estimate the short and long-term costs and CRC-related effects associated with colorectal cancer in first-degree relatives. In this model, 4 health statuses were considered: (1) well, (2) colorectal cancer, (3) well after cancer, and (4) death (Figure 2). The health state was considered as well when there were no cases of CRC. The development of CRC was considered as the state of cancer. The state of well after cancer was considered in the cases who survived for more than 10 years; also, for those who died from CRC, the state of death was defined.
The analysis was carried out using Excel Software and all costs and outcomes were discounted at 3% per annum.
Costs were obtained from several sources for 2018; for example, the cost of molecular testing was obtained by experts. Other costs were collected from the patients’ records admitted in 2 specialized hospitals and also, from the published literature (12). The costs were calculated in the Iranian Rial (IRR) and converted to the US dollar using the 2018 exchange rate (35624 IRR for each USD).
The effectiveness in terms of QALYs and life-years gained (LYG) in relatives was estimated over a lifetime horizon. Utilities for health states were obtained from the literature review. We calculated the QALYs by multiplied the utility index of the health statuses in the number of patients in any status for age groups. Also, the LYG was calculated the avoided number of years of life lost that rise from prevented premature mortality.
To evaluate the validity of the Markov model, One-way sensitivity analyses were carried out for all parameters considered in this study. In order to do the one-way sensitivity analysis, a 20% decrease and increase were applied to all parameters except the discount rate and years of colonoscopy. This range was chosen because it is a common way to use in the sensitivity analysis.
3. Results
This study generated a decision model to compare 5 screening strategies to detect LS cases among the hypothetical 10,000 patients with early-identified CRC in Iran. According to the decision tree analysis, the number of detected LS patients among CRC cases ranged from 124 in strategy 3 to 239 in strategy 2. Overall, testing costs in the CRC patients ranged from 584,332$ in strategy 1 to 1,915,001$ in strategy 5. Strategies 2 and 5 had, therefore, the lowest and highest cost per index mutation detected, resulting in a cost of 2,716 $ and 14,755 $, respectively. Furthermore, the cost of identifying LS in the first-degree relatives in strategies 1 to 5 was 5,770, 4,400, 6,569, 4,669 and 16,439$ per first degree relatives (FDR), respectively. The number of FDRs per CRC patients was considered as five people. Therefore, assuming that a 50% chance of Lynch's mutation was passed to the first-degree relatives, the number of people eligible for regulatory and preventive services in strategies 1 to 5 would be 358, 596, 311, 553 and 324, respectively. In this study, it has been assumed that first-degree relatives, those who inherit the Lynch's syndrome, receive some annual colonoscopy starting from the age of 25; also, the uptake rate is assumed to be 100%. Therefore, as a result of providing the desired surveillance, the total cost, including the cost of colonoscopy and aspirin and the total cost of cancer treatment in strategies 1 to 5, was estimated to be 2,512,390, 3,864,294, 2,282,593, 3,644,790 and 3,630,154$. If there were no prevention, this cost would be approximately twice.
All screening strategies could reduce cancer incidence and death and yield more life-years, as compared with the no-screening strategy. Table 1 displays the incremental costs, incremental effects, and incremental cost-effectiveness ratios [ICERs (QALY) and ICERs (LYG)] in the five screening strategies.
| Strategies | Incremental Cost ($) | Incremental QALY | LYG | ICER1(QALY) | ICER(LYG) |
|---|---|---|---|---|---|
| Strategy 1 | 1,427,775 | 268 | 237 | 5,337 | 6,029 |
| Strategy 2 | 2,054,928 | 446 | 395 | 4,604 | 5,202 |
| Strategy 3 | 1,340,024 | 233 | 206 | 5,763 | 6,511 |
| Strategy 4 | 1,966,129 | 414 | 367 | 4,748 | 5,364 |
| Strategy 5 | 2,645,799 | 249 | 220 | 10,639 | 12,043 |
Abbreviations: LYG, life-year gained; QALY, quality-adjusted life year.
The ICERs ranged from 4,604$ per QALY in the strategy 2 to 10,639$ per QALY in the strategy 5 when QALY was considered as an outcome. Furthermore, when LYG was considered, the ICERs ranged from 5,202$ per LYG in the strategy 2 to 12,043$ per LYG in the strategy 5.
Comparison of the costs and outcomes in different strategies showed that strategy 5 could be ruled out because it was strongly dominated by the strategy 2 (strategy 2 had less cost and better outcomes in comparison to the strategy 5). Strategies 1, 3, and 4 were dominated by strategy 2 because they were less effective and had a higher ICER. However, the strategy 2 was both the most effective and the least costly strategy, with a lower ICER, as compared to the other strategies.
Comparing each strategy with the next least costly strategy showed that the strategy 4 was closer to the strategy 2, and this was followed by the strategy 1 and finally, the strategy 5. The results of this comparison are shown in Table 2.
| Variables | Incremental Cost ($) | QALY | LYG | ICER (QALY) | ICER (LYG) |
|---|---|---|---|---|---|
| S5 - S3 | -1,347,560 | -299 | -298 | 4,506 | 4,520 |
| S3 - S1 | 229,797 | 997 | 1,011 | 231 | 227 |
| S1 - S4 | 1,132,399 | 4,169 | 4,157 | 272 | 272 |
| S4 - S2 | 219,504 | 917 | 918 | 239 | 239 |
Abbreviations: LYG, life-year gained; QALY, quality-adjusted life year.
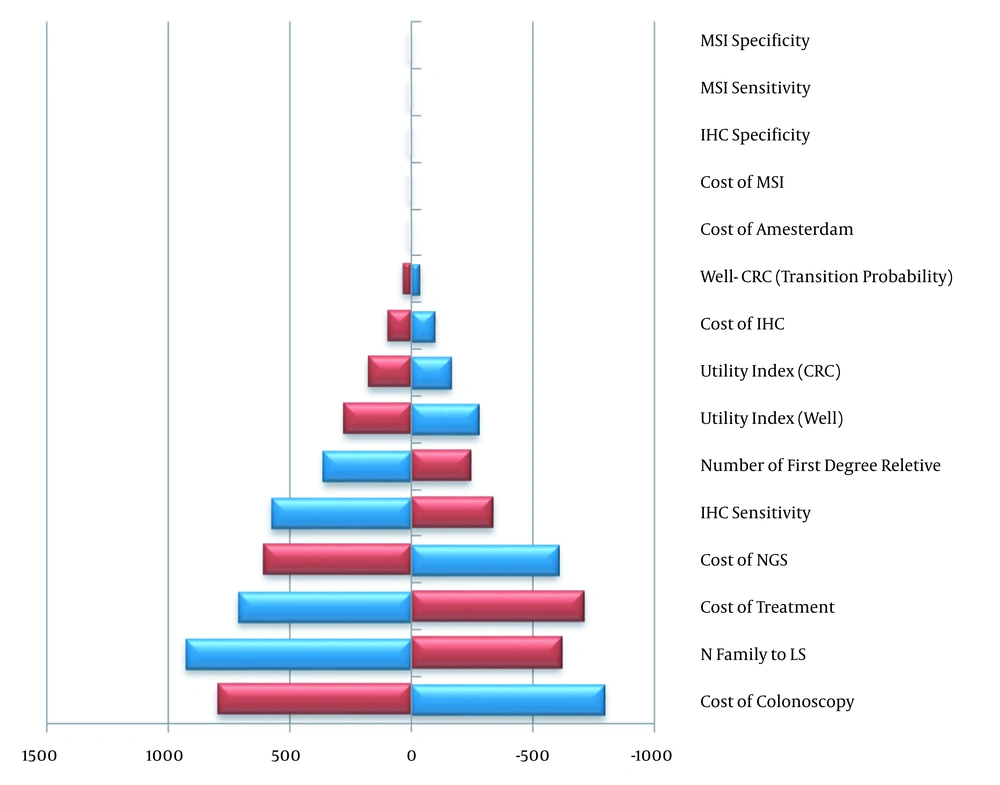
Various variables including sensitivity and specificity of molecular tests, cost of colonoscopy, cost of molecular tests, transition probabilities, the number of the first-degree relatives, CRC treatment cost, the cost of checking the Amsterdam criteria’s, utilities, and the number of families who inherited LS were considered in the one-way sensitivity analysis. The impact of different discount rates (3, 5, and 7%) on the costs and colonoscopy frequency was also examined.
Based on one-way sensitivity analysis, in strategies 1 to 3, IHC sensitivity, the cost of colonoscopy, and MSI sensitivity had the most effect on the results. The same was true for the strategies 4 and 5 with regard to the number of families who inherited LS. Table 3 shows four important variables in each strategy and their range in one way sensitivity analysis. For example, in the strategy 2, which was the most cost-effective, a 20 percent decrease and increase in the variables related to the cost of colonoscopy, the number of families who had inherited LS, the cost of treatment and the cost of NGS led to an ICER range of 1593$, 1543$, 1420$ and 1212$ per QALY respectively.
| Strategy | Rank 1 | Rank 2 | Rank 3 | Rank 4 |
|---|---|---|---|---|
| ICER(QALY) | ||||
| Strategy 1 | IHC sensitivity (6725a - 4662a) | N family to LS (6445a - 4597a) | Colonoscopy cost (4540a - 6133a) | MSI sensitivity (6291a - 4843a) |
| Strategy 2 | NGS cost (5130a - 6397a) | N family to LS(5177a - 4269b) | Colonoscopy cost (3808b - 5401a) | Treatment cost (5314a - 3894b) |
| Strategy 3 | IHC sensitivity (5093a - 6946a) | N family to LS (6979a - 4953a) | Colonoscopy cost (4967a - 6560a) | MSI sensitivity (7549a - 4988a) |
| Strategy 4 | N family to LS (5710a - 4107b) | Colonoscopy cost (3952b - 5545a) | Treatment cost (5458a - 4038b) | NGS cost (4152b - 5344a) |
| Strategy 5 | N family to LS (13114-8990c) | NGS cost (8727a - 12551c) | Number of FDR (12564c - 9356c) | Colonoscopy cost (9860c - 11418c) |
| ICER(LYG) | ||||
| Strategy 1 | IHC sensitivity (7598 - 5267) | N family to LS (7282 - 5194) | Colonoscopy cost (5129 - 6929) | MSI sensitivity (7107 - 5471) |
| Strategy 2 | N family to LS (5849 - 4823) | Colonoscopy cost (4030 - 6101) | Treatment cost (6003 - 4400) | NGS cost (5796 - 7227) |
| Strategy 3 | IHC sensitivity (7847 - 5754) | N family to LS (7884 - 5596) | Colonoscopy cost (5611 - 7411) | MSI sensitivity (8528 - 5636) |
| Strategy 4 | N family to LS (6451 - 4640) | Colonoscopy cost (4464 - 6264) | Treatment cost (6166 - 4562) | NGS cost (4691 - 6038) |
| Strategy 5 | N family to LS (14845 - 10176) | Colonoscopy cost (11162 - 12925) | NGS cost (9879 - 14208) | Number of FDR (12564 - 9356) |
Abbreviations: LYG, life-year gained; QALY, quality-adjusted life year.
a GDP < ICER < 2GDP.
b ICER < GDP.
c 2GDP < ICER < 3GDP.
A 20 percent reduction in colonoscopy costs in the strategy 2 would decrease ICER to 900$ per LYG and 796$ per QALY. Similarly, a 20 percent increase in the number of relatives who have inherited LS could increase LYG by 79 years.
The results of one-way sensitivity analysis are shown in Figure 3 using the Tornado chart. In the graph, the parameters and their range of changes are shown. In this analysis, the effect of changing parameters on the cost-effectiveness results has been evaluated for the QALY outcomes. The results of the sensitivity analysis for the two discount rates of 5 and 7% indicated that ICER was in the range of 8635 - 14651 per QALY and 9837 - 16852 per LYG. Performing a two-year and five-year colonoscopy yielded ICERs of 2379 and 1044$ per QALY. Similarly, 2687 and 1179$ were obtained per LYG.
4. Discussion
The present study is the first one evaluating the cost-effectiveness of different strategies in the patients with colorectal cancer for the detection of the Lynch syndrome in Iran. In this study, 5 screening strategies were applied based on the literature and the expert opinion to evaluate the cost-effectiveness of the screening strategies in identifying LS among the first-degree relatives of the patients who were newly diagnosed with CRC.
The obtained results were similar to the previous studies in terms of the cost-effectiveness of the LS screening. Compared to non-screening, the incidence and mortality rate of cancer were decreased in all strategies. The incremental cost per QALY for the strategy 2 and 5 varied from 4,604 to 10,639$, respectively. Similarly, the incremental cost per LYG varied from 5,202 to 12,043$ for strategies 2 and 5 respectively, compared with no screening.
WHO defines 3 categories of cost-effectiveness: (1) when ICER is less than 1 GDP per capita, it is highly cost-effective; (2) when ICER is between 1 and 3 GDP per capita, it is cost-effective; and (3) when ICER is higher than 3 GDP per capita, it is not cost-effective (16).
The results of this study showed that in all screening strategies, ICER (QALY) was between 1 and 3 Iranian GDP per capita. However, the most cost-effective strategy was the strategy 2 and the most expensive strategy was the strategy 5. Therefore, the combination of IHC and NGS testing could be regarded as the most cost-effective strategy. In the strategies containing only IHC or MSI, ICERs were about 15 to 25 percent less than those containing IHC and MSI tests. Specifically, strategies 2 and 4 were the more cost-effective compared with strategies 1 and 3that combined IHC and MSI.
Regarding the external validity of the results, it should be noted that in the model-based analysis the results should be interpreted considering the assumptions made. We find some similarities and differences in the results of this study and the other studies carried out in this area (13, 14, 16, 17). These differences and similarities could arise from a wide range of factors including the choice of different tests, the structure of strategies, costs identification, the number of first-degree relatives, and the probabilities used in the studies. Similar to the previous studies, we considered family criteria as a first step, based on the family history the tests were performed as an effective factor in the prevention of colorectal cancer.
This study showed that the strategy 2 had the highest total cost, compared to other strategies .This finding was in contrast with the results of the studies by Mvundura et al. and Ying-Erh Chen et al. (13, 16). These studies contained the combined IHC and NGS strategy except for the family criteria.
Among different strategies, the strategy containing direct sequencing had the highest ICER, which was similar to the research conducted by Severin (17). In this study, similar to several previous studies, quality of life was used (13, 22). Considered strategies had a greater impact on the quality of life than the life years gained. In fact, the quality of life had grown more than LYG due to intervention, which was contrary to the results of the previous studies (13, 17, 22).
4.1. Limitation
There were, however, some limitations in this study. It was assumed that 100% of patients and their first-degree relatives could participate in molecular and colonoscopy screening. However, some barriers violate this assumption; these include lack of access to health centers, limited knowledge or awareness, fear of colonoscopy, and its result. Since the health system perspective was used in this study, only direct costs were included in the calculation. Also, the risk of other Lynch-dependent cancers was not considered. The risk of colorectal cancer varies among people carrying different genes in the MMR, but this study considered the same risk due to the complexity of the model and the lack of relevant data. Sensitivity analysis was used to address some of these limitations.
4.2. Conclusion
From the perspective of the Iran health care system, all strategies were cost-effective compared to non-screening. Strategies that began with the Amsterdam criteria and they were followed by IHC or MSI tests, then the NGS test, were the most cost-effective, as compared to non-screening. In fact, the strategy 2 was the most cost-effective one. The cost-effectiveness ratio achieved through the use of NGS testing for the CRC patients without any other molecular testing was close to the upper cost-effective threshold and therefore, had the least cost-effectiveness. The screening would also have many clinical benefits for the first-degree relatives of patients.