1. Introduction
A struma ovarii is a monodermal teratoma, which is exclusively composed of mature thyroid tissue or mature teratomas, with more or less than 50% of thyroid tissue grossly visible. Approximately 7% of mature teratomas are composed of microscopic thyroid tissue, while less than 2% meet the criteria for a struma ovarii. This type of teratoma frequently occurs in the fifth decade of life, and most cases are not diagnosed preoperatively.
Almost 30% of patients with struma ovarii may show features that suggest malignancies (1, 2). These tumors mimic malignant ovarian neoplasms due to the presence of ascites and high cancer antigen-125 (CA-125) levels (3). Therefore, most patients undergo unnecessary extended surgeries. Here, we reported a case of struma ovarii, arising from the ovaries. The evaluations strongly suggested a malignancy due to the presence of ascites, pleural effusion, increased CA-125 levels, and radiological findings before surgery. On the other hand, the histopathological analysis indicated a benign struma ovarii.
2. Case Presentation
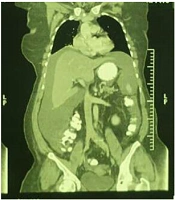
A 54-year-old postmenopausal woman (gravida 8, para 5) with dyspnea and abdominal discomfort was referred to our hospital for evaluating ascites and pleural effusion, suspected of peritoneal carcinomatosis. The physical examination showed massive ascites and diminished breath sounds in the right lung. A transvaginal ultrasound revealed a normal uterus with an enlarged hypervascular right ovary (66 × 42 mm) and severe ascites. A computed tomography (CT) scan with double contrast was performed for the chest, abdomen, and pelvis, which showed moderate pleural effusion in the right lung and severe free fluid buildup in the abdomen with enlarged heterogeneous right adnexa (Figures 1 - 3).
The serum tumor markers were as follows: (1) CA-125 > 500 u/mL, (2) CA19-9: 7 u/mL, and (3) carcinoembryonic antigen (CEA): 0.2 ng/mL. Other laboratory tests, such as complete blood count and thyroid-stimulating hormone (TSH) were normal. Considering the high CA-125 level, massive ascites, pleural effusion, and the enlarged right ovary, peritoneal carcinomatosis was suspected. The physical examination showed massive ascites. The ascites fluid was tapped for a cytological evaluation. An intraperitoneal catheter was implanted to provide self-controlled drainage of the ascetic fluid and reduce abdominal discomfort due to massive ascites until a cytological pathology report was prepared; the results were negative for malignant cells.
Moreover, a diagnostic gastrointestinal endoscopy was performed, the results of which were reported to be normal. Accordingly, the patient underwent exploratory laparotomy, and 1000 mL of clear serous ascetic fluid was evacuated. Although the uterus and the left ovary were unremarkable, the right ovary was enlarged to a size of 60 × 70 mm with an intact capsule; there was no evidence of the involvement of other organs. Besides, a right salpingo-oophorectomy was performed, followed by a frozen-section (FS) procedure, which suggested a struma ovarii (Figure 4).
The patient underwent the total hysterectomy, left salpingo-oophorectomy, and staging surgeries, which included infracolic omentectomy, appendectomy, and pelvic lymph node sampling due to a suspicious pathology report. The patient was discharged from the hospital after 5 days without any complications. After 3 weeks, she showed no dyspnea, and the CA-125 level was below 100 u/mL. The final pathological diagnosis of a struma ovarii was made. Six months after the surgery, she was asymptomatic and annual follow-ups were planned. Written informed consent was obtained from the patient.
3. Discussion
A struma ovarii is a monodermal variant of ovarian teratoma, composed of mature thyroid tissue; thyrotoxicosis is reported in only 5% of these cases (4, 5). In our patient, the thyroid function test was normal. Approximately 8% of strumae present with abnormal vaginal bleeding due to the luteinization of the surrounding ovarian stromal cells. Besides, these tumors can be incidentally discovered on pelvic imaging (1, 5, 6). Malignant ovarian stromal tumors, which occur in 0.3 to 5% of all these tumors, show the histological features of thyroid follicular adenomas (proliferative stromal changes) or papillary carcinomas (1, 7). Also, any extra ovarian spread or tumor infiltration of the ovarian serosa, regardless of histology, is indicative of malignant strumae (1, 7).
The large tumor size, abundant adhesions, and ovarian serosal defects are high-risk features of struma ovarii that may be associated with aggressive behaviors (1, 8). Mildly elevated CA-125 levels are found in up to 30% of these tumors, although in the presence of ascites, this level may be higher (1, 9). Case reports have shown that higher levels of CA-125 were associated with a higher volume of ascites; however, the size of the tumor was not linearly correlated with the CA-125 level (10, 11). In this regard, Lin et al. (cited in Abad et al.) found that CA-125 was expressed by reactive mesothelial cells rather than neoplastic cells in Meigs’ syndrome (12). In our patient, the size of the tumor was small; however, marked ascites resulted in a high level of CA-125.
Meigs’ syndrome is defined as the triad of pleural effusion, ascites, and ovarian fibroma or fibrothecoma. These presentations with other benign ovarian tumors are referred to as pseudo-Meigs’ syndrome, which occurs in 5% of struma ovarii. Both of these syndromes mimic advanced ovarian malignancy (1, 6, 10, 13). Ascites, pseudo-Meigs’ syndrome, and CA125 level reach complete remission following the surgical resection of the ovarian mass, and struma ovarii is not in a high-risk category for malignancy (1, 4, 12).
Unlike most mature teratomas, the stroma of the ovary is not diagnosed preoperatively. Ultrasound is non-specific and generally contains solid components or thick septa in the cystic component similar to an ovarian malignancy (1, 7). On the other hand, successful preoperative diagnosis of a struma ovarii, associated with pseudo-Meigs’ syndrome, has been reported using a combination of 18F-FDG PET/CT and 131I scintigraphy (9). These tumors are classically unilateral, and less than 1% of them are reported to be bilateral. Most struma ovarii cases are solid or solid-cystic on gross examination, and the thyroid tissue can be commonly observed. Microscopically, macrofollicles, and microfollicles contain colloids with scanty stroma, while in some cases, there are fibromatous strumae and fewer follicles, mimicking fibroma or Brenner tumor. A typical struma ovarii contains rare to few mitoses (1, 7). Immunohistochemically, positive thyroid transcription factor 1 (TTF-1), and thyroglobulin are sufficient to confirm the diagnosis of struma ovarii (1, 7, 8).
3.1. Conclusions
A struma ovarii may be misdiagnosed as advanced ovarian cancer due to ascites, high CA125 levels, and imaging features. A preoperative examination is not helpful for diagnosis, and an intraoperative diagnosis based on frozen-section analysis results in unnecessary extensive surgeries. Complete excision of this tumor is curative, and its prognosis is excellent. It seems that long-term postoperative follow-up is not required, except in high-risk cases. However, patients may be sometimes scheduled for yearly follow-ups with CA-125 measurement and ultrasonography.