1. Background
Sarcomas, a heterogeneous group of tumors of mesenchymal origin, are relatively rare types of malignancies with a fairly high mortality rate (1). These tumors account for less than 1% of all adult cancers and constitute approximately 21% of all diagnosed pediatric solid tumors (2). Sarcomas can affect any age group with a higher incidence in young and adolescent population and their occurrence is not limited to a specific anatomic site (2, 3). These tumors represent a wide range of malignancies that complicate their study, diagnosis, and treatment. In this regard, more than 70 distinct histologic sarcoma subtypes have been characterized (4). Although these malignancies can originate from any location in the body, commonly are categorized into 2 main groups: (1) soft tissue sarcomas (STS), and (2) bone sarcomas (BS) (2). As the name implies, STS mainly affect the soft tissues of the body including different muscles, blood vessels, joints, nerves, and fat as well as skin tissues. However, BS can involve bone and cartilage structures throughout the human body.
There are numerous pieces of evidence indicating that cancer incidence and its associated mortality are rapidly growing worldwide (5). It has been demonstrated that the distribution trends of different types of cancers, including sarcomas, substantially varies across countries and within each county as well (5). In this regard, the analysis of 45,568 incident cases diagnosed during 1995 to 2002 by a multi-institutional international study in Europe revealed a total crude incidence of sarcomas of 5.6 per 100,000 individuals per year with an estimated 27,908 new cases per year in the EU27 countries (6). Furthermore, based on the results of the Surveillance, Epidemiology, and End Results (SEER) program from 2002 to 2015 in the United States, a total number of 78,527 cases of sarcomas were identified which showed an overall incidence of 7.1 cases per 100,000 people (7). Another study that investigated the incidence and mortality of sarcomas in Shanghai from 2002 to 2015, demonstrated an age-standardized the incidence rate (ASIR) of 3.4 per 100,000 people (8). It is obvious that there is a difference in incidence of sarcomas among these countries. The reasons for this are quite complex and may depend on different environmental, lifestyle, socioeconomic, genetic, and cultural factors (1, 9, 10). Collectively, via better knowledge of the incidence, prevalence, and distribution patterns of sarcomas, it will be possible to provide better health cares to improve outcomes for patients who suffering from these malignancies and implement proper measures to steer away from the risk factors involved in sarcomas development.
2. Objectives
According to the literature, only a limited number of studies have been conducted in Iran to investigate the epidemiology and incidence of cancer. Likewise, little is known about the epidemiological distribution and incidence of different types of sarcomas at the national and provincial levels in Iran. Therefore, the present study was aimed at providing data on the incidence rates and distribution patterns of morphological subtypes and primary sites of both soft tissue and bone sarcomas in a 6-year period (2009 - 2014) in the total population of Iran.
3. Methods
In this study, the data on patients with cancer who were diagnosed with sarcoma from 2009 to 2014 was retrieved from the Iranian National Cancer Registry (INCR) which collects cancer registry data from 33 provinces nationwide. Provincial data were collected by local cancer registries under the observation of Chief Medical Universities in Iran, covering somehow the total Iranian population (more than 90%). The collected data were submitted to INCR, pooled together and de-identified, and were analyzed for this study. The provided data included patients’ names, ID numbers, age, gender (female or male), date of birth, residential province, and year of diagnosis, as well as tumor location, morphology, and grade of the tumor. The various aspects of this study complied with the ethical standards and were approved by the Ethical Committee of Shahid Beheshti University of Medical Sciences. Of note, the included subjects were those only who meet the morphologic criteria for soft tissue sarcoma (STS) and bone sarcoma (BS) based on the classifications purposed by the International Agency for Research on Cancer (IARC) (11). Accordingly, after data cleaning, 14630 cases out of initially 19868 identified patients were found to be eligible and included in the present study.
All the cancer cases were categorized based on the morphological types and primary site of the tumor and were coded according to the 3rd revision of the standard International Classification of Diseases for Oncology (ICD-O-3) and WHO classification 2018 (12-14). The morphological subtypes of the soft tissue sarcoma were identified by ICD-O-3 M codes 8800-8935, 8910, 8920, 8940, 8950–8959, 8963–8964, 8990–8991, 9020–9044, 9120–9133, 9150, 9170, 9180, 9231, 9240, 9251, 9260, 9364–9372, 9540, 9560–9571, 9580–9581, etc. The morphological subtypes of the bone sarcoma were identified by M codes 8800–8920, 9040–9044, 9120–9133, 9150, 9170, 9180–9250, 9260–9261, 9310, 9364, 9370, 9540–9581 and etc. The ICD-O-3 codes C00-C80 (except C40.0–41.9) and C40.0–41.9 were assigned to STS and bone tumors, respectively based on their anatomic site of origin.
The incidence rates were calculated as the number of new cases in a period of 1 year divided by the estimated resident mid-year Iranian population reported by the Iranian Bureau of Statistics and expressed as the number per 100,000 people. Furthermore, age-standardized incidence rates (ASIR) for both STS and BS (with 95% CI) were calculated by adjusting for the age distribution of the total Iranian and Segi's World Population. Proportions of STS and BS were calculated by dividing the number of cases with STS or BS by the total diagnosed cases. The proportions of each morphological subtype were also determined as a percentage of all STS or BS diagnoses for each year from 2009 to 2014. Additionally, incidence rates were presented by age at diagnosis and were grouped into 3 age cohorts from 0 to 14, 15 - 64, and 65+ years. For further analysis, the patients were grouped in 5 years age cohorts from 0 to 4, 4 - 9, and 10 - 14 up to 80 years. Collectively, the identified sarcoma cases were examined for 118 different morphologies, 117 different tumor locations, and for 6 different tumor grades including score 0, grade 1, 2, 3, 4, and 9, with regards to patients’ sex and abovementioned age cohorts, and finally, the respective incidence rates were obtained according to these variables. All analyses were conducted using Statistical Package for the Stata Corp. 2019 (Stata Statistical Software: release 16. College Station, TX: Stata Corp LLC).
4. Results
A total number of 14630 cases with either STS or BS were identified nationwide between 2009 and 2015. The cases were examined for 117 different locations, 118 different morphologies, and 6 different tumor grades including scores 0, grade 1, 2, 3, 4, and 9, in this study. The overall median age of the patients was 47 [IQR 28–62] years. However, the median age of STS cases was 50 [IQR 33–64], and the median age of BS patients was 25 [IQR 17–41] years. Stratification of cases into three age categories (0 - 14, 15 - 64, and 65 +) showed that most of the cases were aged between 15 and 64 (71.3%) (Table 1). As shown in Table 1, 47.8 and 52.2% of the sarcoma cases were females and males, respectively. According to the results, regardless of the age and sex of the patients, almost similar incidence rates were observed in each year over the study period, however; it is obvious that the incidence rate was slightly decreased in 2011 (Table 1). Based on our findings (Table 2), bone sarcomas comprised 16.47% of the cases, while soft tissue sarcomas were accounted for 83.53% of detected cases during the 6 years of analysis. As shown in Table 1, the annual crude incidence rates by gender and the total annual crude incidence rates were calculated in this study. The total combined crude incidence over the study period was obtained 3.2 per 100000 people. The age-standardized incidence rates were higher in males than females (Table 2). According to Table 2, the majority of the cases (10948) were of grade 9 (unknown/undifferentiated grade). Regardless of tumors with grade 9, tumors with grade 3, 1, 2, 4, and score 0 had the highest frequencies with the mentioned order. As presented in Table 3 S1, the sarcoma, osteosarcoma, leiomyosarcoma, liposarcoma, spindle cell sarcoma, and Ewing sarcoma were responsible for the majority of the phenotypes. Furthermore, based on the results summarized in Table 3 S2, the connective tissue of the lower limb, long bone of lower limb, skin and uterus, specifically formed the 4 most common primary tumor sites, respectively. However, bronchus and lung, bone (except limbs), long bones joint and cartilages, and pelvic bones were found as primary sites for sarcoma development with the least frequencies.
| Diagnosis (y) | Cases (n) | Age Median [IQR] | Age, % [95% CI] | Gender, % Male [95% CI] | CR | Incidence Rate per 100,000 [95% CI] | |
|---|---|---|---|---|---|---|---|
| 15 - 65 year | > 65 year | ||||||
| 2009 | 2,146 | 45 [26 - 61] | 70.9 [69.0, 72.8] | 20.0 [18.4, 21.8] | 51.3 [49.2, 53.4] | 2.91 | 3.1 [2.8, 3.5] |
| 2010 | 2,047 | 45 [28 - 62] | 71.9 [69.9, 73.9] | 19.9 [18.2, 21.7] | 53.1 [50.9, 55.2] | 2.75 | 2.7 [2.4, 3.0] |
| 2011 | 2,723 | 46 [28 - 62] | 71.5 [69.8, 73.2] | 22.0 [20.5, 23.6] | 52.6 [50.7, 54.5] | 3.62 | 3.9 [3.5, 4.3] |
| 2012 | 2,686 | 47 [28 - 62] | 70.9 [69.2, 72.7] | 21.1 [19.6, 22.7] | 52.9 [51.0, 54.8] | 3.53 | 3.7 [3.3, 4.1] |
| 2013 | 2,590 | 48 [29 - 62] | 71.7 [69.9, 73.4] | 20.7 [19.2, 22.4] | 51.3 [49.4, 53.3] | 3.36 | 3.5 [2.9, 4.0] |
| 2014 | 2,438 | 48 [30 - 62] | 71.2 [69.4, 72.9] | 21.4 [19.8, 23.0] | 51.5 [49.5, 53.5] | 3.12 | 3.2 [2.7,3.7] |
| Overall | 14630 | 47 [28 - 62] | 71.4 [70.6 , 72.1] | 20.9 [20.3, 21.6] | 52.1 [50.3, 53.9] | 3.22 | 3.2 [3.0,3.5] |
Incidence of Sarcomas in Iran 2009 - 2014 by Diagnosis Year (n = 14,630)
| Variables | No. (%) | ASIR per 100,000 | ||
|---|---|---|---|---|
| Female | Male | Both | ||
| Overall | 14630 (100) | 3.0 [2.8, 3.2] | 3.3 [3.1, 3.5] | 3.2 [3.0, 3.5] |
| Bone sarcoma | 2410 (16.47) | 0.37 [0.33, 0.40] | 0.51 [0.47, 0.56] | 0.44 [0.41, 0.47] |
| Soft tissue sarcoma | 12220 (83.53) | 2.6 [2.4, 2.8] | 2.8 [2.6, 3.0] | 2.7 [2.6, 2.9] |
| Time period | ||||
| 2009 | 2,146 (14.67) | 2.9 [2.5, 3.3] | 3.3 [2.9, 3.7] | 3.1 [2.8, 3.5] |
| 2010 | 2,047 (13.99) | 2.5 [2.2, 2.9] | 2.8 [2.5, 3.2] | 2.7 [2.4, 3.0] |
| 2011 | 2,723 (18.61) | 3.6 [3.2, 4.1] | 4.2 [3.7, 4.7] | 3.9 [3.5, 4.3] |
| 2012 | 2,686 (18.36) | 3.4 [3.1, 3.8] | 4.0 [3.5, 4.5] | 3.7 [3.3, 4.1] |
| 2013 | 2,590 (17.70) | 3.3 [2.8, 3.8] | 3.6 [2.9, 4.3] | 3.5 [2.9, 4.0] |
| 2014 | 2,438 (16.66) | 3.0 [2.6, 3.5] | 3.3 [2.7, 3.9] | 3.2 [2.7,3.7] |
| Grading | ||||
| 0 | 41 (0.3) | < 0.01 [< 0.01, 0.01] | 0.02 [< 0.01, 0.03] | 0.01 [< 0.01,0.22] |
| 1 | 1302 (8.9) | 0.28 [0.24, 0.31] | 0.27 [0.23, 0.30] | 0.27 [0.24, 0.30] |
| 2 | 601 (4.1) | 0.12 [0.10, 0.14] | 0.13 [0.10, 0.15] | 0.12 [0.10, 0.14] |
| 3 | 1473 (10.1) | 0.28 [0.24, 0.32] | 0.36 [0.31, 0.41] | 0.32 [0.28, 0.35] |
| 4 | 265 (1.8) | 0.04 [0.03, 0.06] | 0.05 [0.04, 0.07] | 0.05 [0.04,0.06] |
| 9 | 10948 (74.8) | 2.21 [2.11, 2.44] | 2.50 [2.32, 2.74] | 2.42 [2.23,2.51] |
Numbers, Percentages, and Age-Standardized Incidence Rates of Sarcomas Based on the Type, Diagnosis Year, and Tumor Grades
| Variables | No. (%) | ASIR per 100,000 | ||
|---|---|---|---|---|
| Female | Male | Both | ||
| Histological group | ||||
| Sarcoma | 1,284 (8.78) | 0.29 [0.27, 0.31] | 0.32 [0.30, 0.34] | 0.30 [0.28, 0.32] |
| Osteosarcoma | 1,038 (7.10) | 0.21 [0.18, 0.24] | 0.28 [0.24, 0.32] | 0.25 [0.22, 0.27] |
| Leiomyoma sarcoma | 959 (6.56) | 0.37 [0.34, 0.40] | 0.11 [0.09, 0.13] | 0.24 [0.21, 0.26] |
| Liposarcoma | 929 (6.35) | 0.19 [0.17, 0.21] | 0.26 [0.24, 0.28] | 0.23 [0.21, 0.25] |
| Spindle cell sarcoma | 904 (6.18) | 0.17 [0.14, 0.19] | 0.22 [0.18, 0.27] | 0.19 [0.17, 0.22] |
| Ewing sarcoma | 725 (4.96) | 0.12 [0.10, 0.14] | 0.17 [0.15, 0.19] | 0.15 [0.13, 0.17] |
| Malignant fibrous histiocytoma | 652 (4.46) | 0.12 [0.14, 0.18] | 0.20 [0.17, 0.23] | 0.16 [0.14, 0.18] |
| Chondreosarcoma | 592 (4.05) | 0.11 [0.08, 0.13] | 0.17 [0.14, 0.20] | 0.14 [0.12, 0.16] |
| Dermatofibrosarcoma | 580 (3.96) | 0.11 [0.09, 0.13] | 0.11 [0.08, 0.13] | 0.11 [0.09, 0.12] |
| Gastrointestinal stromal sarcoma | 447 (3.05) | 0.09 [0.07, 0.11] | 0.11 [0.09, 0.14] | 0.10 [0.08, 0.12] |
| Kaposi sarcoma | 443 (3.03) | 0.05 [0.03, 0.06] | 0.17 [0.13, 0.20] | 0.11 [0.09, 0.13] |
| Desmoplastic small round cell tumor | 392 (2.68) | 0.08 [0.06, 0.10] | 0.11 [0.08, 0.14] | 0.09 [0.07, 0.12] |
| Mesothelioma malignant | 384 (2.62) | 0.07 [0.05, 0.09] | 0.08 [0.06, 0.10] | 0.08 [0.06, 0.09] |
| Malignant peripheral nerve sheath tumor | 384 (2.62) | 0.07 [0.05, 0.09] | 0.09 [0.07, 0.11] | 0.08 [0.06, 0.09] |
| Malignant tumor spindle cell type | 365 (2.49) | 0.08 [0.06, 0.11] | 0.09 [0.06, 0.11] | 0.09 [0.07, 0.10] |
| Synovial sarcoma | 342 (2.34) | 0.08 [0.06, 0.09] | 0.11 [0.09, 0.13] | 0.09 [0.08, 0.11] |
| Fibro sarcoma | 308 (2.1) | 0.07 [0.60, 0.09] | 0.07 [0.06, 0.09] | 0.07 [0.62, 0.08] |
| Giant cell sarcoma except of bone | 266 (1.82) | 0.04 [0.03, 0.05] | 0.08 [0.05, 0.10] | 0.06 [0.04, 0.07] |
| Primary tumor location | ||||
| Connective tissue of lower limb | 1,500 (10.25) | 0.28 [0.24, 0.31] | 0.34 [0.30, 0.38] | 0.31 [0.28, 0.33] |
| Long bone of lower limb | 1,320 (9.02) | 0.21 [0.19, 0.24] | 0.28 [0.25, 0.32] | 0.25 [0.22, 0.27] |
| Skin | 1,110 (7.59) | 0.20 [0.17, 0.23] | 0.29 [0.25, 0.32] | 0.24 [0.22, 0.27] |
| Uterus | 904 (6.18) | 0.38 [0.34, 0.42] | 0.00 [0.00, 0.00] | 0.19 [0.17, 0.21] |
| Unknown primary sites | 742 (5.07) | 0.13 [0.10, 0.15] | 0.16 [0.13, 0.19] | 0.15 [0.12, 0.17] |
| Connective tissue of upper limb | 638 (4.36) | 0.10 [0.08, 0.11] | 0.18 [0.15, 0.21] | 0.14 [0.12, 0.15] |
| Connective and other soft tissues | 439 (3.00) | 0.07 [0.05, 0.09] | 0.12 [0.10, 0.15] | 0.10 [0.08, 0.12] |
| Heart, mediastinum and pleura | 430 (2.94) | 0.06 [0.05, 0.08] | 0.11 [0.09, 0.14] | 0.09 [0.07, 0.11] |
| Connective tissue of pelvis | 417 (2.85) | 0.07 [0.06, 0.09] | 0.09 [0.07, 0.12] | 0.08 [0.07, 0.10] |
| Retro peritoneum | 406 (2.78) | 0.08 [0.06, 0.10] | 0.12 [0.09, 0.14] | 0.10 [0.08, 0.12] |
| Connective tissue of thorax | 352 (2.41) | 0.08 [0.06, 0.10] | 0.12 [0.09, 0.14] | 0.10 [0.08, 0.12] |
| Pelvic bones | 331 (2.26) | 0.04 [0.05, 0.07] | 0.07 [0.06, 0.09] | 0.06 [0.05, 0.07] |
| Long bones, joints and cartilages of upper limbs | 314 (2.15) | 0.04 [0.03, 0.06] | 0.07 [0.05, 0.08] | 0.05 [0.05, 0.06] |
| Bone except limbs | 313 (2.14) | 0.05 [0.02, 0.07] | 0.07 [0.05, 0.09] | 0.06 [0.04, 0.07] |
| Bronchus and lung | 295 (2.02) | 0.06 [0.04, 0.08] | 0.06 [0.04, 0.08] | 0.06 [0.05, 0.07] |
The Age-Standardized Incidence Rate of Sarcomas Based on Histological Subtypes and Primary Tumor Locations Through the 6-Years Study Period
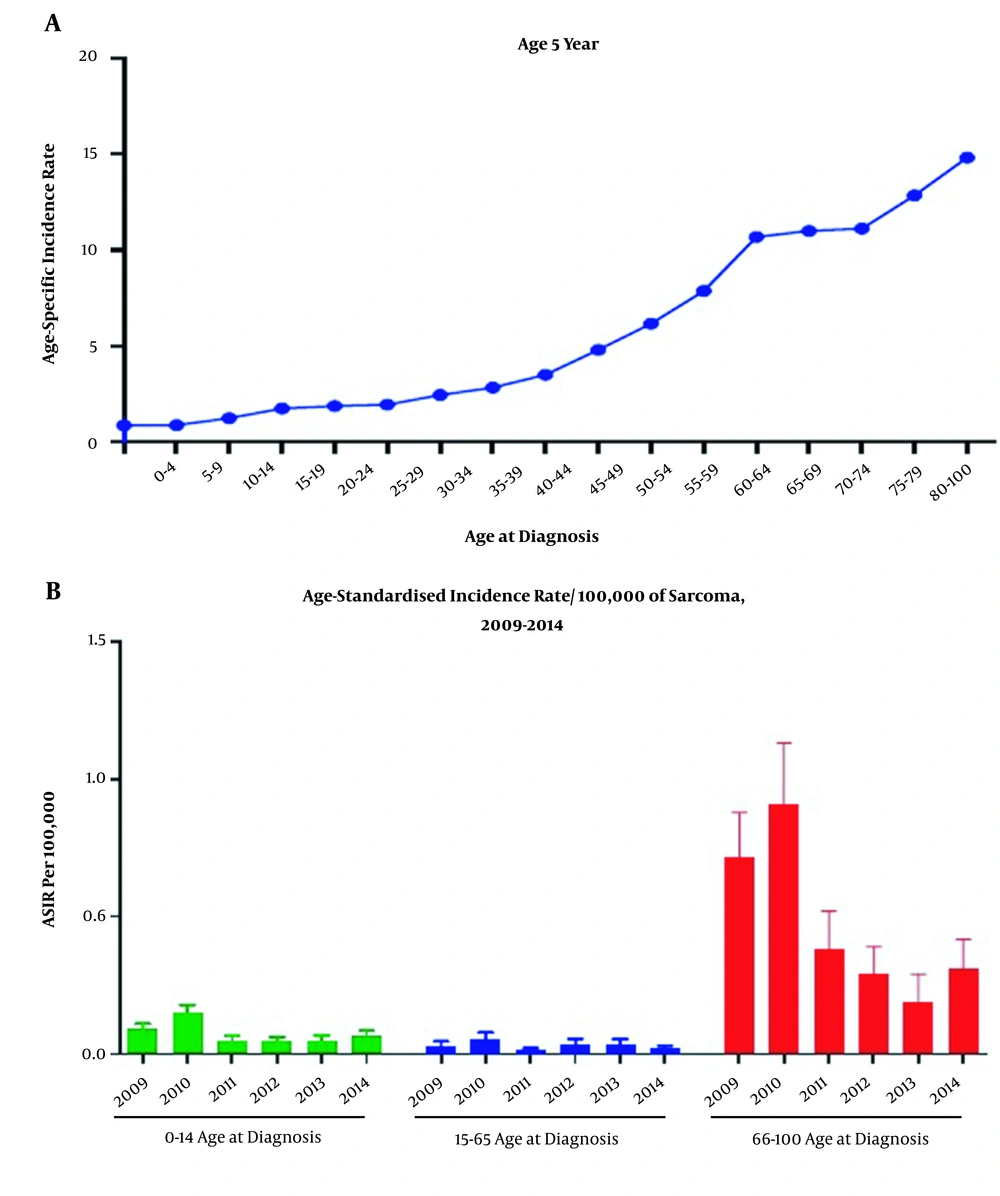
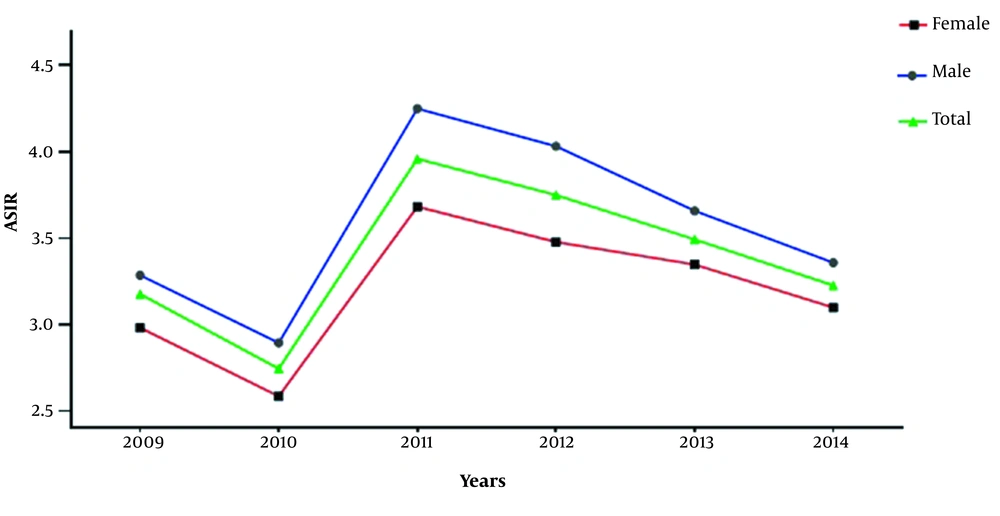
The age-specific incidence rates revealed that the incidence of sarcoma was increased with age (Figure 1A). We also examined the morphological and topographical distribution patterns of both STS and BS based on the age-standardized incidence rate (ASIR) analysis. Accordingly, the overall ASIR of STS and BS was found to be 2.7 [95% CI 2.6, 2.9] and 0.44 [95% CI 0.41, 0.47] per 100,000 people per year, respectively for the study period (Table 2). Based on the results, the ASIR for STS in male and female were 2.8 [95% CI 2.6, 3.0] and 2.6 [95% CI 2.4, 2.8] per 100,000 individuals, respectively, while the ASIR for BS was 0.51[95% CI 0.47, 0.56] and 0.37 [95% CI 0.33, 0.40] in males and females, respectively (Table 2). The trends of ASIR of sarcoma are also illustrated in Figure 2. According to these results, a male preponderance was observed, and the total ASIR increased from 2.7 [95% CI 2.4, 3.0] in 2010 to 3.9 [95% CI 3.5, 4.3] in 2011 per 100000 Iranian population. However, the total ASIR was only slightly increased from 2009 to 2014 from 3.1 [95% CI 2.8, 3.5] to 3.2 [95% CI 2.7, 3.7] per 100000 people.
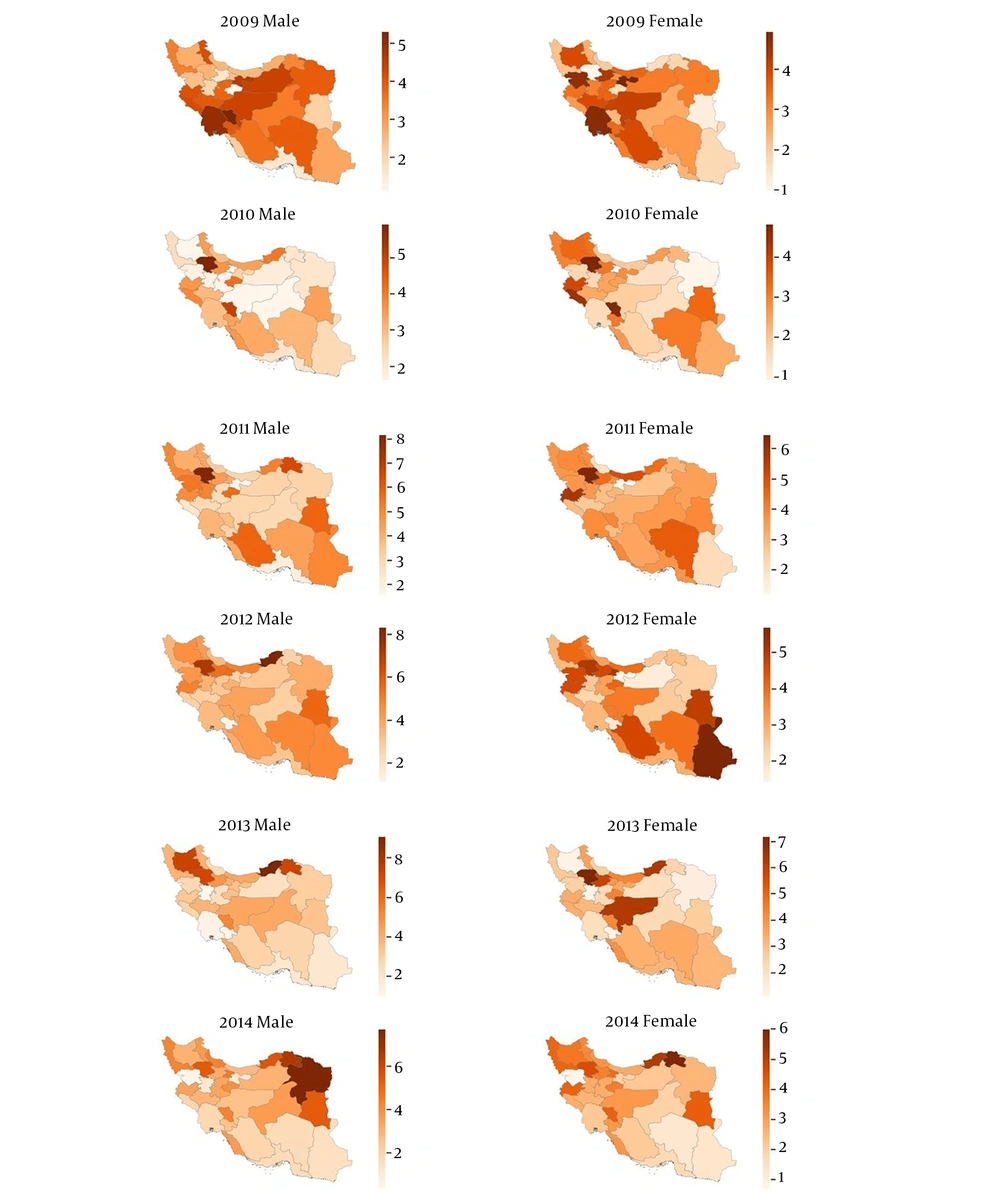
The provincial age-standardized incidence rate of sarcoma is also provided in Figure 3. Although the distribution patterns of the sarcoma patients varied at the provincial level over the study period, the highest ASIR for sarcomas belonged to Khuzestan, Kohgiloyeh and Boyer-Ahmad, Isfahan, Tehran, Fars, and Khorasan Razavi provinces; however, ASIR of sarcomas was at the lowest level in Booshehr, Zanjan, and Hormozgan provinces in the 6-years of analysis. The details of the age-adjusted incidence rates of sarcoma in each province by the year are provided in S3.
As presented in Figure 1A, the total incidence of sarcoma is increased by increasing age. Furthermore, based on Figure 1B, the ASIR of sarcoma was higher among the > 65 years group compared to the other age categories. However, since the category aged 15 to 64 contributes to the main part of the total population, thus the number of sarcoma cases aged 15 to 64 is higher than the other 2 categories.
5. Discussion
In the present study, we performed a population-based epidemiological analysis of both STS and BS patients in Iran from 2009 to 2014 for the first time.
Numerous studies have been conducted to study sarcoma incidence worldwide. Although it has been shown that sarcomas account for only 1% of all tumors, but the incidence rate of sarcoma varies in different countries or different regions within a country (15). In this regard, the results of the PARECARE project, an investigation conducted in the EU27 countries, revealed a total crude incidence rate of 5.6 per 100,000 individuals for sarcomas in the subjected European countries (6). More recently, the results of the SEER program (2002 - 2015) showed a crude incidence of 7.1 per 100,000 in the United States (7). Another study conducted in Shanghai assessed the incidence of sarcoma from 2002 to 2015 and demonstrated a crude incidence rate of 5.3 per 100,000 individuals (8). As it is obvious, the incidence rates of sarcoma in the above-mentioned studies are substantially higher than that corresponding rate found in the present study (crude incidence of 3.2). However, in line with the findings of the PARECARE project and the results of Shanghai study, approximately 84 and 16% of the cases were soft tissue sarcomas and bone sarcomas, respectively. A male preponderance was noted in our study. The literature review showed remarkable inconsistency in the male to female predominance and vice versa in sarcoma incidence. In this regard, the incidence of sarcoma was found to be similar in males and females in Shanghai, China (8). However, a study conducted in Taiwan revealed a male predominance in the incidence of bone sarcoma (16). In the contrast, female predilection has been reported by the studies conducted in EU27 countries, Ireland, and China (6, 17, 18). The female predilection in these studies has been attributed to the higher number of incidents that occurred in feminine genital organs (uterus) and breasts and more patients with subtypes like leiomyosarcoma, which occurs mainly in female genital organs and uterus.
Different incidence rates for STS and BS have also been reported similar to the corresponding rates reported for sarcomas of all types. In this study, the total combined crude incidence rate, the crude incidence rates for STSs and BS were found 3.2, 2.7, and 0.44 per 100,000 individuals, respectively. Moreover, the age-standardized incidence rates of 2.8 and 2.6 per 100000 for STS, and 0.51 and 0.37 per 100000 for BS were obtained for males and females, respectively. Similar to our results, age-standardized rates of 2.8 and 0.6 were found for STS and BS, respectively in the Shanghai study (8). However, different age-standardized rates have been reported by the studies from EU27 countries (3.3 - 4.7/100000), Taiwan (5.62/100000), Switzerland (4.47/100000), Ireland (4.48/10000), and three European regions (4.58/100000 for males and 5.12/100000 for females) (6, 17, 19-21).
Additionally, there was an obvious difference in incidence rates of sarcomas based on their morphology and topology. As presented in Table 3, S1 and S2, limbs were the most common locations for sarcoma development. Similar findings have also been reported by the PARECARE project and in Taiwan, Ireland, and China studies (6, 17-19). The abdomen and retroperitoneum were the second most common site for sarcoma among our patients, which was in line with the China study (18). Regardless of primary tumor sites, there are various histological subtypes of sarcomas. In this regard, more than 70 histotypes have been attributed to soft tissue sarcomas (9). Understanding the distribution of sarcoma histotypes as well as primary sites for tumor development is necessarily essential for better management of patients with tumor (22). For example, a unique histological subtype of sarcoma in different sites may need entirely different treatment modalities. Initially, we found that sarcoma of NOS was the most common histotype which was followed by osteosarcoma, leiomyosarcoma (uterus and non-uterus leiomyosarcoma), liposarcoma, spindle cell sarcoma, and Ewing sarcoma. However, the age-standardized analysis showed that sarcoma of NOS, leiomyosarcoma, liposarcoma, spindle cell sarcoma, osteosarcoma, and malignant fibrous sarcoma was the most common histological subtypes (Table 3). Likewise, sarcoma of NOS was found as the most common histological subtype in studies from United States, Shanghai, Switzerland, China, and Austria which was followed differentially by leiomyosarcoma, liposarcoma, gastrointestinal sarcoma, or malignant fibrous sarcoma (7, 8, 18, 20, 23). It is obvious that the histological classification based on age-standardized analysis was closely compatible with the findings of the abovementioned studies. It seems that this can be due to that in the mentioned studies only the age-standardized analysis was performed.
It has been shown that the tumor grade has an important impact on patients’ outcomes and directly associated with overall survival and disease-free survival in patients with cancer. Moreover, tumors with known grades can be managed better than those with unknown grades. Therefore, sarcoma patients in our study were classified based on the tumor grades. Strikingly, most of the cases in this study were of unknown/undifferentiated grades (74.83%), which was followed by grade 3 (10.07%), grade 1 (8.90%), grade 2 (4.11%), grade 4 (1.81%), and grade 0 (0.28%), respectively. The same results were also obtained by age-standardized analyses. In the same way, in the SEER study, 42.2 % of the identified patients were of unknown/unspecified grades (7). However, the results of the SEER program revealed that sarcomas with unknown grades are not a proportional mixture of other grades but instead may represent disproportionately rare subtypes of sarcoma tumors that are being inadequately graded and subsequently, possibly inadequately managed.
We also calculated sarcoma incidence analysis at the provincial level. As shown in Figure 1, the highest age-standardized the incidence rates for males and females were observed in Khuzestan, Kohgiloyeh and Boyer-Ahmad, Isfahan, Tehran, Fars, and Khorasan Razavi. Accordingly, there is a remarkable difference in incidence of sarcomas among different provinces in Iran. Similar to our findings, different rates for sarcoma incidence have been shown in different provinces of Austria (23). However, the ASIR of sarcoma in different provinces of Iran were higher than the corresponding rates obtained by the mentioned study. Since the composition of the Iranian population is heterogeneous (consists of different races including Fars, Turk, Arab, Baloch and Kurd), and their lifestyles and potentially genetical traits differ from each other, thus this could probably influence the difference in incidence rates of different provinces. Despite the population heterogeneity and genetic-environmental variables which might potentially affect the provincial incidence rates, it can also arise from the different capability of the provincial registration systems. For example, Bushehr and Sistan-Baluchestan are poor provinces; thus the low incidence rate of sarcoma observed in these states could be due to the less capable of provincial cancer registry. Therefore such confounders can result in the underestimated incidence rates and registration facilities should be improved to accurately evaluate the incidence of malignancies throughout the country. Other variables including cultural, and economical status, as well as environmental, and climate conditions may also have effect on different incidence rates (10). Moreover, we found that trends in incidence rate only slightly changed over the study period (Table 2 and Figure 2), which was also demonstrated in the Taiwan study. In the contrast, the incidence rates were significantly changed in the Australian study over the 10 years study period. Based on the observation (Figure 2), a drop was observed in the incidence rate in 2010, while somehow continued to increase in the next years. Since in the early years that the Iranian Cancer Registry launched, the enrollment of patients were mainly pathology-based registry, while it was shifted toward population-based registry in the latter years, therefore the drop and rise in the incidence trends might be indicative of the accuracy of cancer statistics.
As mentioned previously, the patients in this study stratified into 3 age categories (0 - 14, 15 - 64, and > 65). Our findings showed that incidence age-standardized incidence rates were increased with the age, with a peak at ≥ 65 years (Figure 1). In accordance with these findings, higher incidence rates were observed in the > 65 age group in Australia, Shanghai, Japan, EU27 countries, and Ireland (6, 8, 17, 24, 25). Taken together, these findings indicated that, although there are some differences in the incidence of sarcomas between countries, somehow a similar pattern of incidences maybe existed among different age groups. Moreover, it seems that because of different physiological changes and probably compromised immune system, elderly people are at higher risk to develop sarcoma.
5.1. Conclusion
The present study is the first study that has provided a comprehensive understanding of the incidence, histological subtypes, and primary tumor locations of STS and BS nationwide and at a provincial level in Iran over a 6-year period (2009 - 2014). The crude and age-standardized incidence rates were compared to the values reported by other studies. Based on the findings, the incidence rates for STS and BS were comparable to those of international incidence rates. The distribution of morphological and topographical patterns was somehow similar to those of international patterns. Remarkable differences were observed for sarcoma incidence between different provinces. Further studies should be done to investigated patients’ survival as well as etiological factors which may influence sarcoma incidence in different provinces.