1. Background
Smoking is one of the causes of preventive diseases and death in the world (1). The number of people who die because of using nicotine is more than other preventable agents that cause death (2). Smoking is one of the main 8 causes of death in the world. It has been reported that 5 million people die because of smoking around the world (2). Despite the health benefits of smoking cessation, the level of avoidance with the present medications is low. Lung cancer (LC) is a leading cause of cancer morbidity and mortality worldwide.
LC is still the most common cancer worldwide in terms of the number of newly diagnosed cases (1.8 million cases, 12.9% of all cases) and mortality rate (1.6 million deaths, 19.4% of all cases) (3). One of the predisposing factors for LC is smoking.
People smoke cigarettes for different reasons such as anger, irritability, depression, anxiety, and difficulty concentrating. Studies have shown that the experience of anxiety and negative emotions play an important role in continuing smoking (4). There are currently 3 common therapies that are approved by the Food and Drug Administration (FDA) (to reduce the withdrawal symptoms of smoking cessation, which include bupropion (labeled with Wellbutrin and Zyban), which is an anti-depressant drug that is from the amino tonic group and it is a Norepinephrine-Dopamine reuptake inhibitor (NDRI) and is a nicotine antagonist. Varenicline/Chantix is a topical agonist for acetylcholine receptors and Nicotine replacement therapy (NRT). Although 70% of smokers are reluctant to smoking cessation, these treatments have only been useful for 15% of the cases (2).
Metformin (Glucophage), as an anti-diabetic drug, is the first-line therapy for diabetes, which reduces total intracellular cholesterol by stimulating AMP-activated protein kinase (AMPK) activity (5). It is estimated that more than 50 million patients with diabetes use this medication daily (6). In addition, studies showed that metformin had significant effects on anxiety reduction and depression in patients with Polycystic Ovary Syndrome (PCOS) (7). Also, the results of the meta-analysis of Zhou et al. (8) showed that metformin has been effective in weight controlling that resulted from antipsychotic drugs compared to placebo. In contrast, the results of a study by Wiwanitkit and Wiwanitkit (6) showed that metformin consumption in a patient with diabetes mellitus has been associated with complaints of insomnia symptoms.
Metformin has been used in seizure management (9). In this regard, the results of Pirnia et al.’s (10, 11) study showed that anti-seizure drugs such as Topiramate have some effects on the stimulant drug cravings reducing. It seems that the AMPK pathway is a common point for the effects of metformin on seizure and nicotine withdrawal reduction. Studies showed that the AMPK pathway in the hippocampus is activated after chronic nicotine consumption and rapidly reversed by nicotine withdrawal (2). Increasing Phosphorylated AMP-activated protein kinase (pAMPK) levels and subsequent reduction of AMPK signaling lead to reduce the anxiety symptoms that are resulted from nicotine withdrawal. The results of Brynildsen et al.’s (2) study showed that the AMPL activation can be used as a therapeutic goal in smoking cessation.
Although the use of pharmacological interventions in smoking cessation has a long history, limited studies have reported the use of metformin. In addition, the research literature suggests that metformin may be involved in inhibiting the growth of cancer cells. Due to the dual role of metformin in smoking cessation and inhibition of cancer cell growth, this study is of clinical importance.
2. Objectives
This study was conducted to evaluate the effects of metformin on reducing cigarette withdrawal syndrome and increasing nicotine abstinence in patients with LC. The primary outcomes include metformin efficacy on cigarette withdrawal syndrome in 8 components and secondary outcomes include urinary cotinine levels and eCO level.
3. Methods
3.1. Patients and Design
The current study was a double-blind placebo-controlled trial with a 6-month follow-up that registered in Iranian Registry of Clinical Trials (IRCT: IRCT20160218026628N1). Data were collected from February 2018 to May 2019.
Among the people with LC who were referred to 6 smoking cessation centers in Tehran Province, 60 participants were screened by a Respondent-driven Sampling (RDS) and obtaining a cut-off point that equals to 3 or more than 3 in the Fagerstrom Test for Nicotine Dependence (FTND).
Eligibility criteria included age range of 18 - 65 years, diagnosis of malignant neoplasm of lung based on the International Classification of Diseases (ICD)-10 (C34), the average smoking rate of at least 5 cigarettes in a day (PCD) for at least 1 year, avoiding from smoking in the enrolment phase for at least seven days, body mass index (BMI) at the range of 18 - 35, not using any psychiatric medication or receiving psychological interventions in the 3 months that lead up to the study, and desire to reduce or stop smoking and obtain informed consent. Exclusion criteria included the diagnosis of diabetes mellitus or high blood pressure or any side effects such as seizure or dizziness, liver, and kidney disease, severe infection, lactic acidosis susceptibility, epilepsy, or any condition that may reduce the seizure threshold.
After obtaining inclusion criteria and obtaining informed consent, they entered into the research process. Seven participants were excluded due to side effects. Finally, 27 participants in the metformin group and 26 participants in the placebo group (wait-list control (WLC)) were randomly assigned through block randomization (BR) (Figure 1).
3.2. Sample Size
The sample size was 60 subjects. This study was performed concerning the assumption of Z =1.96, d = 0.25, a = 0.05, and also the power of test 1-B = 0.84 and possible sample fall in below formula: (12).
Taking into account the possibility of dropping the subject, 10 participants were added to the sample size and 60 participants were considered.
3.3. Intervention
Metformin was started at a daily dose of 500 mg (one 500 mg tablet) in the first week and administered at a daily dose of 1000 mg (Aria, Iran) for 12 weeks to the participants in the experimental group. The drug and placebo were administered by two nurses who were unaware of the study groups. All participants received brief behavioral compliance enhancement treatment (BBCET).
Demographic Checklist: The researcher used this questionnaire to collect personal information such as age, education, marital status, occupation, and the duration of nicotine use (13).
The Structured Clinical Interview for DSM-4 (SCID-4): It is a Clinical Interview that is used to diagnose dysfunctions of axis 1 based on DSM-IV (14, 15).
Smoking history questionnaire: This questionnaire was used to evaluate the years of smoking and the average of daily smoking during the last week.
Cigarette Withdrawal Scale (CWS-21): This instrument has 21 items and assesses withdrawal syndrome in 6 components of depression-anxiety, cravings, irritability-enthusiasm, increased appetite-weight, insomnia, and difficulty in concentrating. It is a multidimensional valid test (Cronbach's alpha = 83-96) of the cigarette withdrawal syndrome that is sensitive to changes over time and is a predictor of relapse in smoking (16).
Urinary cotinine assessments and exhaled carbon monoxide (eCO) level: To ensure nicotine abstinence, urinary cotinine assessments and eCO levels were used. A Micro CO detector was also used to estimate the alveolar eCO concentration in parts per million (ppm). The urine cotinine levels were analyzed, using a gas chromatography-mass spectrometry (GC-MS). GC-MS is an analytical method that combines the features of gas-chromatography and mass spectrometry to identify different substances within a test sample.
Anthropometric measurements (weight and body fat measurements): Concerning the dependence of metformin effects on body weight and body fat, all participants were screened in terms of two indices of Seca Supra plus 720 (Seca, Hamburg, Germany), and the data were analyzed by Bioelectrical Impedance Analysis (BIA) (17).
3.4. Statistical Analysis
The data were analyzed for normalization, using Shapiro–Wilk test. The distribution of anthropometric and clinical variables was abnormal in both groups. The generalized estimation equation (GEE) was used to test the difference between the two groups in nicotine withdrawal and urinary cotinine and eCO level at multiple intervals. The cut-off point of the Fagerstrom test was 5 for nicotine dependency. Also, urinary cotinine concentrations of 50 ng/mL and 8 ppm of eCO were the cut-off points to confirm smoking abstinence (according to Moreno-Coutiño and Villalobos-Gallegos, (18). Demographic characters were compared with the chi-square test in the two groups, and qualitative findings including side effects were analyzed by Atlas-Ti5 software. The reduction of cigarette withdrawal syndrome during 12 weeks of treatment was considered the primary outcomes and urinary cotinine assessments and eCO levels once a week were considered the secondary outcomes. At the beginning of the study and in the form of multi-baselines, the variables were evaluated. Changes in lactic acidosis were also evaluated as metformin's possible side effect. The data were analyzed by statistical package for the social sciences (SPSS) software (version 22) and the significance criterion was 0.05.
3.5. Ethical Approval
This study was part of a research project approved by the Medical Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.CRC.REC.1399.035). All interviews were digitally recorded and copied from the words and coded. All stages of the research were performed after obtaining informed consent from the patient and based on the Declaration of Helsinki (DoH) (19).
4. Results
Table 1 demonstrates the demographic characteristics of the participants in the study.
| Characteristic | Experimental a | WLC a | P-Value |
|---|---|---|---|
| Age | 33.87 ± 7.31 | 35.21 ± 7.03 | NS |
| Cigarettes per day | 19.01 ± 4.22 | 17.53 ± 6.55 | NS |
| Age of the first cigarette | 15.21 ± 3.24 | 18.21 ± 3.83 | NS |
| Regular smoking onset | 19.95 ± 3.42 | 18.59 ± 3.27 | NS |
| Years as a smoker | 18.09 ± 6.08 | 16.47 ± 5.22 | NS |
| Previous quitting attempts | 1.03 ± 0.84 | 1.15 ± 0.62 | NS |
| Previous treatments | 1.49 ± 0.52 | 1.99 ± 0.64 | NS |
| Urine cotinine at baseline(ng/mL) | 2653.1 ± 712.03 | 283.949 ± 629.81 | NS |
| Baseline exhaled CO (ppm) | 13.71 ± 4.29 | 15.47 ± 6.11 | NS |
| Sex, % | |||
| Female | 29.83 | 33.93 | < 0.05 |
| Male | 66.29 | 69.95 | NS |
| Marital status, % | |||
| Married/cohabiting | 28.89 | 33.31 | < 0.05 |
| Separated/divorced | 23.51 | 19.07 | < 0.05 |
| Never married | 49.23 | 45.99 | < 0.05 |
| Years of education, % | |||
| Under the diploma | 29.21 | 26.07 | NS |
| Bachelor | 52.29 | 49.93 | NS |
| Master's degree | 22.17 | 16.25 | < 0.05 |
| Ph.D. degree | 1.83 | 2.25 | NS |
Abbreviations: NS: no significance, WLC: wait-list control, SD: standard deviation.
a Values are presented as mean ± SD unless otherwise indicted.
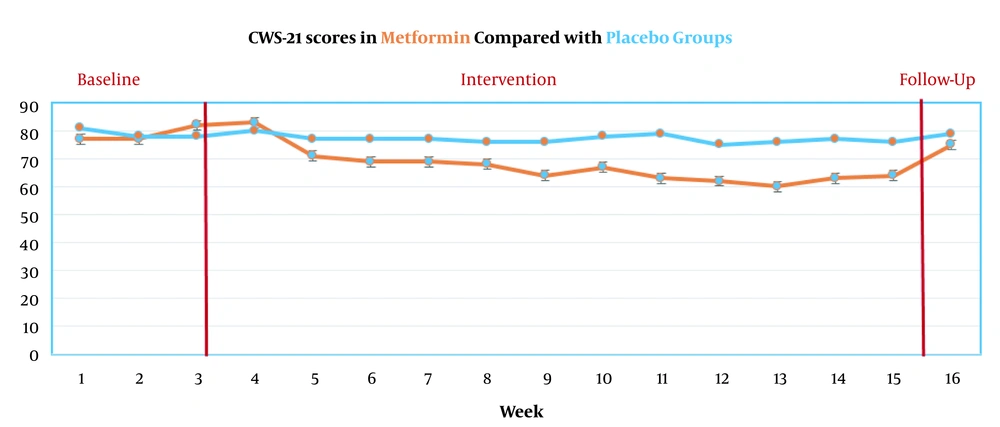
GEE was used to test the difference between the two groups in nicotine withdrawal and urinary cotinine and eCO level at multiple intervals. The distribution of the scores of CWS during the 16 evaluation stages is presented in Figure 2.
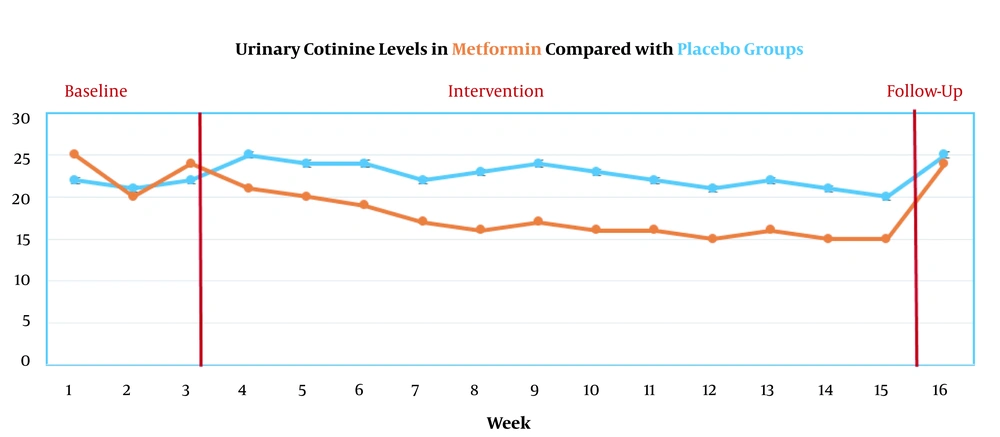
The primary outcomes showed that the metformin group had significant effects on the improvement of depression, anxiety, craving, irritability, and appetite, difficulty in concentrating, appetite-weight, and insomnia during the 12-week treatment (all P’s < 0.05). The results showed that only craving scores remained constant until the 6-month follow-up (P < 0.04) (Figure 2). Urinary cotinine levels during the 16 evaluation stages are presented in Figure 3.
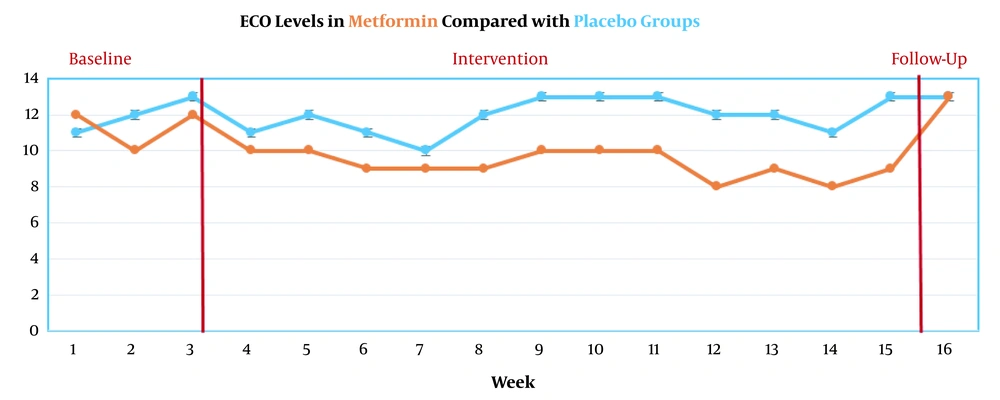
Secondary outcomes showed that urinary cotinine levels significantly decreased in the metformin group (P < 0.05). However, this reduction did not remain constant until the 6-month follow-up (P = 0.71) (Figure 3). ECO levels during the 16 evaluation stages are presented in Figure 4.
ECO levels significantly decreased in the metformin group (P<0.05). However, this reduction did not remain constant until 6-month follow-up (P = 0.97) (Figure 4).
In this study, in a participant lactic acidosis that was shown, in the 8 weeks, 4 participants had nausea and at 12th-week indigestion due to metformin in two participants were observed, and they were excluded from the study.
5. Discussion
This study was a double-blind placebo-controlled trial that was performed to evaluate the effects of metformin on reducing cigarette withdrawal syndrome and increasing nicotine abstinence in patients with LC. The primary outcomes showed that metformin had a significant effect on improving depression, anxiety, craving, irritability, appetite, difficulty in concentrating, appetite-weight, and insomnia in the experimental group. But only the craving scores reduction remained constant until the 6-month follow-up. In line with these results, the results of a study conducted by Brynildsen et al. (2) showed that metformin, with increased PAMPK and consequent reduction in AMPK signaling, reduces anxiety symptoms due to nicotine withdrawal. Also, Erensoy et al. (7) showed that metformin had significant effects on anxiety and depression reduction in polycystic ovary syndrome patients. A part of the results of the current study shows insomnia in the metformin recipient group. Despite the limited research evidence in this regard, the results of the study of Wiwanitkit and Wiwanitkit (6) showed that metformin use in patients with diabetes mellitus has been associated with complaints of insomnia symptoms. These results also showed that the metformin group showed a significant decrease in the appetite-weight component. However, due to the effect of metformin on stimulation of AMPK activity and total intracellular cholesterol reduction, these results were not far behind, but it was expected that the mechanism of this process would be different in non-diabetic individuals. In line with these results, the results of Zhou et al.’s (8) meta-analysis showed that metformin has some effects on weight controlling that resulted from antipsychotic drugs compared to placebo.
Secondary outcomes of this study showed that the levels of urinary cotinine and eCO significantly decreased in the metformin group, although this decrease did not remain constant in both levels until the follow-up year. These results are in line with the results of Brynildsen et al.’s (2) study, which showed that metformin, with increased PAMPK levels and decreased AMPK signaling, reduces anxiety symptoms due to nicotine withdrawal and can reduce smoking. In this regard, the results of Svicher et al.’s (4) study showed that the experience of anxiety and negative emotional states play a role in continuing smoking.
Strengths include originality, new insights, and the double-blind placebo-controlled trial design with several time points. This study had some limitations. This study included people who were volunteered for smoking cessation; therefore, there is the possibility of involvement of motivational variables. Secondly, the effect of metformin was examined just on Iranian smokers; so, the results are not generalizable. Eventually, the number of potential participants who did not participate was not recorded.
In general, this study was conducted to evaluate the effect of metformin on the reduction of nicotine withdrawal syndrome and increasing nicotine abstinence. The results of this study showed that metformin has a significant effect on improving depression-anxiety, craving, irritability, appetite, and difficulty in concentrating. But, it was associated with adverse effects on appetite-weight and insomnia in smokers during the abstinence phase. Only craving scores remained constant until the follow-up year, and other components return to the baseline level. Metformin also significantly reduced urinary cotinine levels and eCO. However, this reduction does not remain constant at both levels until the 6-month follow-up. Performing a clinical trial on the effects of metformin on reducing drug use can be a good route to understand the mechanisms that are involved in managing consumption.