1. Background
Cancer is the first and the second leading cause of death in developed and developing countries, respectively. Gastric cancer (GC) is known as the most common cancer and the second leading cause of cancer death in the world (1). GC is one of the most common malignancies worldwide. This disease is multifactorial disease, and many factors such as infectious, environmental and genetic factors are involved in its occurrence (2). The effectiveness of early prevention programs depends on the knowledge available about the GC risk factors. Numerous risk factors such as occupational and environmental exposure (3) have been found for GC.
Over the past few decades, researchers have drawn attention to the potential threats of devices that produce weak electromagnetic fields in homes, workplaces, hospitals, or even the electronic tools that people carry with them, (4). In the living environment, the most common frequency used by devices is between 50 - 60 Hz and their current intensity varies between 6 and 10 amps. Therefore, the intensity of the magnetic field obtained from these devices varies between 0.1 and 8 mT, depending on the current intensity and the distance from the device (5). They can lead to various biological effects depending on the density, frequency, and duration of their radiation (6). Electromagnetic waves have been reported to cause breast cancer in men (7) and various types of cancer in Norwegian workers (8).
One of the genes in which mutations cause GC is the phosphatase and tensin homolog (PTEN), which is responsible for tumor suppression and is located on chromosome 10q23.3 (9). This gene induces apoptosis and inhibits tumor cell proliferation, cell binding control, migration, tumor invasion (10), and decreased cell differentiation (9). Progression of GC has been shown to associate with inactivation of PTEN through genetic mutation (11), heterozygous elimination (12), and promoter hypermethylation (13). Therefore, the inactivation of this gene in GC plays an important role in tumor progression. A novel type of non-coding RNAs is known as circular RNAs (circ-RNAs) have played as critical regulators in various cancers such as GC (14). The biological processes, cellular functions, and pathological processes such as tumoral cell invasion, migration, and proliferation are affected by circ-RNAs (15). In GC, circ-RNAs have been shown to affect gene expression, and there have even been advances in the use of circ-RNAs as diagnostic and prognostic tools and therapeutic targets (16). The circ-CDR1as circ-RNA is located on chrX: 139865339-139866824. circ-CDR1as has been considered as a risk factor for hepatocellular carcinoma (17). circ-CDR1as has been shown to act as an oncogene via the stimulation of tumor cell proliferation and cell migration in a variety of cancers (18).
2. Objectives
Since the importance of PTEN and circ-CDR1as in cell apoptosis and proliferation and their role in tumor progression, the aim of the present study was to investigate their expression level changes following the exposure of various electromagnetic fields in human GC cell line (AGS).
3. Methods
3.1. Preparation and Treatment of GC Cell Line
The AGS cell line and HU02 were obtained from the National Genetic Resources Center of Iran and then the cells were cultured according to the instructions. AGS cell line was cultured in Ham's F12 medium containing 10% fetal bovine serum (FBS) (Gibco, USA). Normal HU02 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco, USA) containing 10% FBS, 2 mL of L-glutamine, and 1% penicillin/streptomycin (Gibco, USA). The cells were exposed to electromagnetic fields at a magnetic flux density of 0.25, 0.5, 1, and 2 mT continuously and discontinuously with 1 hour interval time for 18 hours. The control and exposed groups were incubated in a constant condition of temperature, humidity, and CO2.
3.2. Exposure System
A solenoid cylinder with a diameter of 12 cm, height of 30 cm, and 1200 turns was used to produce electromagnetic fields (6). An AC power supply (TDGC2, 220v, 50 - 60 Hz Delta International Electric, Shanghai, China) was applied to produce various electromagnetic intensities using city power with a frequency of 50 Hz (19). The electromagnetic field generated inside the solenoid is uniform and in the direction of the cylindrical axis. The solenoid cylinder was horizontally placed at the center of the CO2 incubator and flasks of cells were placed at the middle of the cylinder.
3.3. Cell Survival Assay
The 3-(4,5-dimethylthiazoyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to measure cell viability. For this purpose, the supernatant was firstly separated and removed by centrifugation at 2500 rpm. The MTT solution was then added to the tubes containing tumor and normal cells in a mitochondrial isolation buffer, after which the tubes were incubated for 4 hours at 37°C. After 4 hours of incubation, 200μL dimethylsulfoxide (DMSO) was added to each tube and shaken well to dissolve all formazan crystals. Then the solution was transferred to a 96-wells plate and after one hour the adsorption was read at 560 nm by micro plate reader (Synegry HT, USA).
3.4. RNA Extraction and cDNA Synthesis
RNA was extracted by a chloroform-ethanol method using lysis solution (Trizol sigma Aldrich) and its quality and quantity were evaluated using nanodrop (Thermo Fisher Scientific, USA) and electrophoresis on 1.5% agarose gel. Oligo (dT) or random primers and cDNA synthesis kit (BioFACT, South Korea) were used for cDNA synthesis. The instructions of the kit manufacturer for cDNA synthesis were followed. The polymerase chain reaction (PCR) temperature program used for cDNA synthesis was 65°C for 5 min, 55°C for 30 min 95°C for 10 seconds. The synthesized cDNAs were stored at a -20°C freezer.
3.5. Primer Design and Real-Time-PCR
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an internal control. The primer design was done using primer 3 software and then examined using IDT and Gene runner softwares. The primer sequences of GAPDH, PTEN, and circ-CDR1as are shown in Table 1.
| Genes | Sequences |
|---|---|
| circRNAcdr1as | |
| Forward | 5′-TCAACTGGCTCAATATCCATGTC-3′ |
| Reverse | 5′-ACCTTGACACAGGTGCCAT-3′ |
| PTEN | |
| Forward | 5′-AGTCGCCTGTCACCATTTC-3′ |
| Reverse | 5′-ATTCTCTGGATCAGAGTCAG-3′ |
| GAPDH | |
| Forward | 5′-GCACCGTCAAGGCTGAGAAC-3′ |
| Reverse | 5′-GGATCTCGCTCCTGGAAGATG-3′ |
Abbreviations: PTEN, phosphatase and tensin homolog; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
The real-time polymerase chain reaction (real-time-PCR) timing and temperature program started at 95°C for 30 seconds for cDNA denaturation. In the next step, 40 cycles of 95°C for 5 seconds and 60°C for 31 min were performed. In the next step, the temperature cycle of 95°C for 15 seconds, 60°C for 30 seconds, and 95°C for 15 seconds was used. The cycle of threshold (CT) was determined by Bioneer software. The relative expression of genes to the housekeeping gene was calculated by measuring the delta threshold cycle value (ΔCT) for each sample. Delta delta cycle value (ΔΔCT) was then calculated from the difference between ΔCT of exposed tumor or normal cells and the control group. The fold change of gene and miRs expression was then calculated by the 2−ΔΔCT formula. The PCR efficiency was calculated for the instrument and considered in the formula. The CV% for 10 separate measurements of RNA expression was 11.7.
3.6. Statistical Analysis
Data analysis was performed using SPSS software version 25. The results are shown as mean ± SD. To compare the significant differences among the groups, a two-way analysis of variance and Tukey Post hoc test were used. The level of probability for the significant differences among the groups was considered as P < 0.05. The correlation between PTEN and circRNA-CDR1as was also investigated.
4. Results
4.1. Viability Test
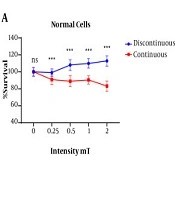
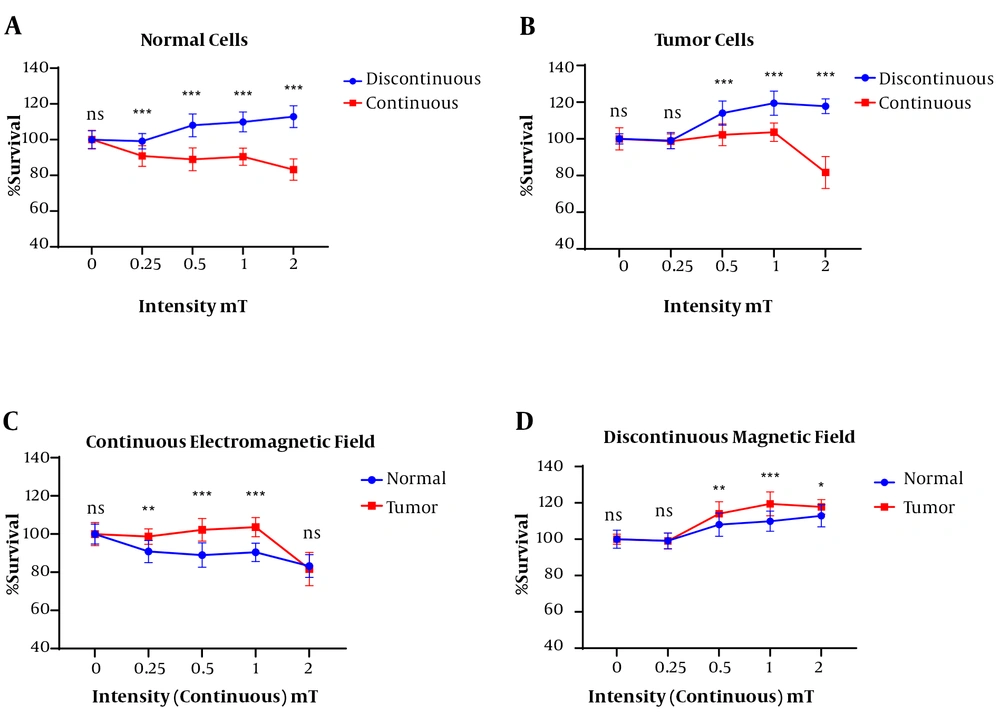
The MTT test results showed a significant difference between the continuous and discontinuous exposed normal cells (HU02) in term of mean percentage of survival rate. The normal cells in the discontinuous magnetic fields group had a higher survival rate than those in the continuous magnetic field group. In addition, with increasing the intensity of the magnetic field in the continuous state to 2 mT, the mean survival percentage was significantly decreased compared to other intensities and the lowest survival rate was observed. The normal cells viability percentage was not decreased in different intensities of discontinuous magnetic fields (Figure 1A).
Between continuous and discontinuous exposure at different magnetic flux densities, a significant difference was seen in the AGS survival rate (P < 0.0001). Unlike the discontinuous, the exposure of AGS cells to continuous electromagnetic fields at 2 mT approximately showed a 20% decrease in survival rate (Figure 1B).
A significant difference in the exposed normal cells in all the continuous magnetic field intensities was observed compared to the tumor cells with the same condition in terms of the mean percentage of cell viability. The exposed tumor cells showed 100% survival up to 1 mT intensity of the continuous magnetic field, but with increasing magnetic field to 2 mT, the tumor cell survival was greatly reduced. The normal cells showed a decrease in cell survival when exposed to a continuous magnetic field at 0.25 mT intensity, which was approximately equal to 1 mT, but when the magnetic field was increased to 2 mT, there was a sharp decrease in cell survival. The results showed that the normal cells have a higher sensitivity to the continuous magnetic field than those of the tumor cells (Figure 1C).
The results of the present study showed that in the exposed the tumor cell with a discontinuous magnetic field resulted in a significant increase in cell survival compared to the exposed normal cell at the same condition (Figure 1D).
4.2. PTEN and Circ-CDR1as Gene Expression
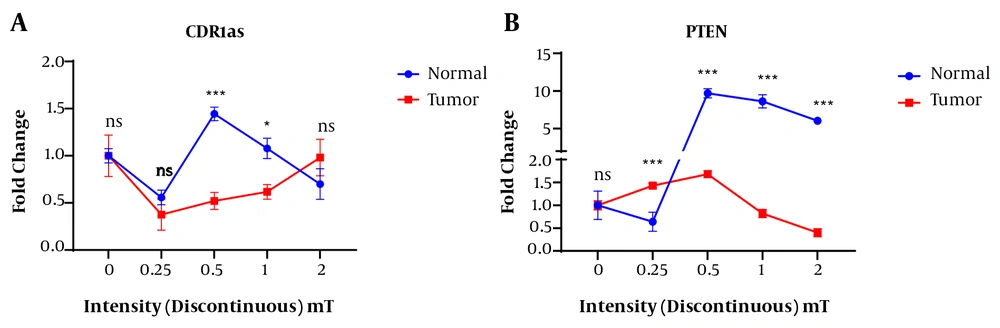
The expression level of circ-CDR1as in the normal cells was increased under the exposure to the discontinuous electromagnetic fields of 0.5 (P < 0.0001) and 1 mT (P < 0.05) intensities when compared to the tumor cells. However, no significant difference in the expression of this gene was observed in the normal and tumor cells at the discontinuous electromagnetic field of 0.25 and 2 mT (Figure 2A).
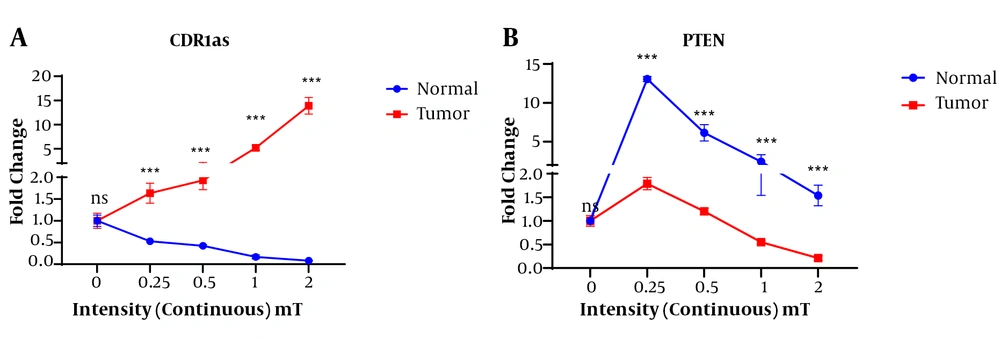
In the present study, the continuous magnetic field led to the change in the expression levels of circ-CDR1as and PTEN genes. Whit continuous exposure, the expression level of CDR1as in AGS cells was increased with increasing the intensity of the magnetic field so that the highest expression level of this gene was obtained at the magnetic flux density of 2 mT. However, the tumor cells showed a decreased expression level of circ-CDR1as with increasing magnetic field intensity and the lowest expression of this gene was observed in 2 mT continuous magnetic field intensity (Figure 3A).
The highest expression of the PTEN gene was observed in the exposed normal cells at the intensity of 0.25 mT continuous magnetic field. The PTEN was significantly expressed in the normal exposed cell at the various intensities of continuous magnetic field compared to the tumor cells exposed to the same magnetic field. However, the highest expression was observed at the intensity of 0.25 (Figure 3B).
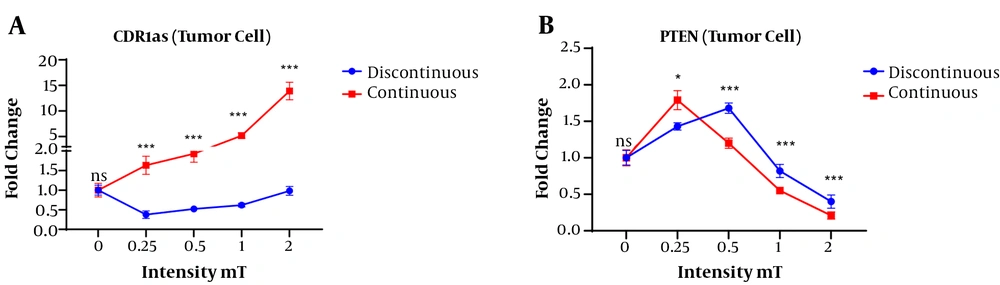
In tumor cells, a significant difference was observed in the expression of CDR1as gene depending on the applied continuous or discontinuous magnetic fields (Figure 4). The highest expression of the CDR1as gene was observed in the exposed tumor cells with continuous magnetic fields with intensities of 2 mT and 1 mT. The mean expression of the CDR1as gene in the exposed tumor cells showed a significant difference at the continuous magnetic intensities of 0.5, 1, and 2 mT. However, the expression of the CDR1as in the tumor cells showed no significant difference when they discontinuously were exposed to various magnetic fields (Figure 4A).
The exposed tumor cells at 0.25 mT intensity of continuous magnetic fields resulted in a significant up-regulation of the PTEN gen compared to the exposed tumor cells at 0.25 mT intensity of discontinuous magnetic fields. In contrast, in the discontinuous magnetic field, the exposed tumor cells at intensities of 0.5, 1, and 2 resulted in the up-regulation of PTEN compared to the same intensity of continuous magnetic field (Figure 4B).
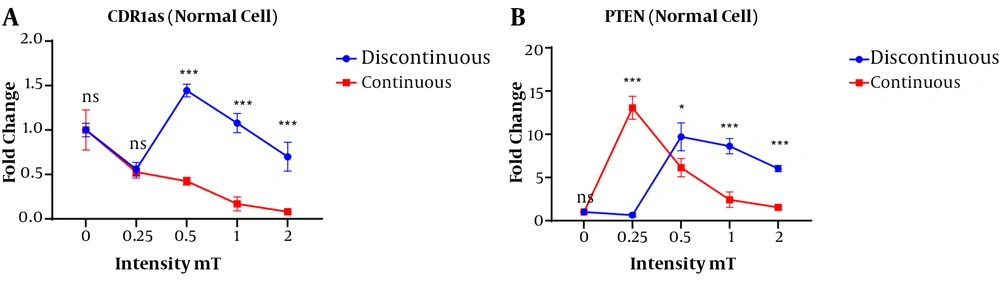
In normal cells, a significant difference was observed in the expression of circ-CDR1as and PTEN genes between the continuous and discontinuous magnetic fields with different intensities (Figure 5). The CDR1as gene expression in the exposed normal cells at the discontinuous magnetic field was higher than that of the exposure to the continuous magnetic field. In the normal cells, the highest and lowest expression of this gene belonged to the intensity of 0.5 mT discontinuous and a continuous magnetic field of 2 mT magnetic field, respectively (Figure 5A).
In the normal cells, the highest expression of the PTEN gene was obtained under continuous magnetic field at an intensity of 0.25 mT and then with increasing the intensity of the magnetic field, the expression of this gene was decreased. In discontinuous conditions, the highest PTEN gene expression was observed in the 0.5 mT magnetic field (Figure 5B).
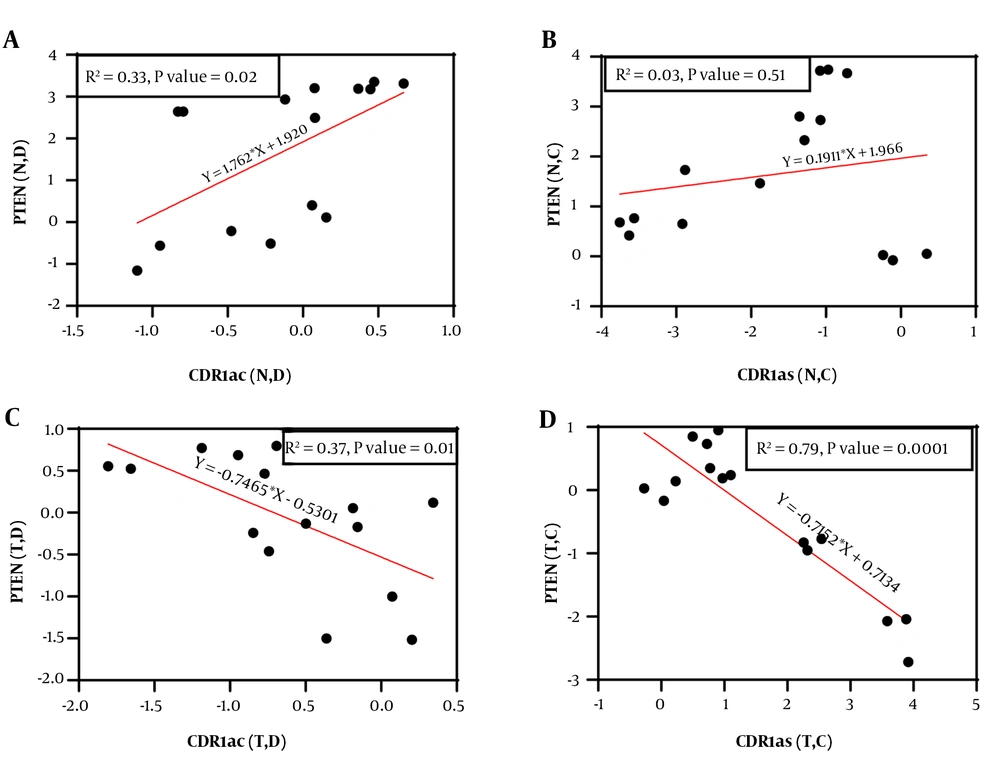
A significant positive correlation (r = 0.574, 95% CI = 0.08786 to 0.8396, P = 0.025) was observed between the expression of PTEN and circ-CDR1as genes in the exposed normal cells to discontinuous magnetic field. However, no significant correlation was observed between the expression of PTEN and circ-CDR1as genes under continuous magnetic field application (P = 0.514). Interestingly, in the AGS cell line, there was a significant negative correlation (r = -0.611, 95% CI = -0.8556 to -0.1441, P = 0.015) (r = -0.8916, 95%CI = -0.9637 to -0.6982, P < 0.0001) between the expression of PTEN and circ-CDR1as genes by applying both discontinuous and continuous magnetic field (Figure 6).
5. Discussion
Attention to electromagnetic exposure as a targeted tumor therapy is increasing recently (20). In addition, due to modern living conditions, exposure to electromagnetic fields caused by various devices is inevitable, which can be created from natural sources or by man-made sources such as diagnostic equipment, nuclear power plants, television receivers, and the like. In the current study, the effect of continuous and discontinuous electromagnetic fields exposure at various intensities on AGS cell viability, PTEN, and CDR1as genes in the normal and GC cell lines was aimed. The effects of electromagnetic fields on the biological system have been extensively studied (21, 22). However, due to the uncertainty of the results and the uncertainty of the mechanism of action of these fields, more studies are needed.
A different effect of discontinuous and continuous exposure of electromagnetic fields on the viability of both normal and tumor cells was one of the main findings of the present study. On the other hand, the exposed normal and tumor cells to discontinuous electromagnetic fields resulted in the increase of cell survival rate in both normal and tumor cells. In contrast, the exposure of continuous electromagnetic field showed no effect on the viability of the normal and tumor cells at a magnetic flux density of 0.25, 0.5, and 1 mT. In addition, a decrease in viability of both normal and tumor cells was seen at a 2 mT continuous magnetic field. Finally, the viability of the exposed tumor cells at both continuous and discontinuous electromagnetic fields showed higher than that of the normal cells. Based on cell viability results, it can be concluded that only continuous exposure at 2 mT reduces the viability of tumor cells. It has been recently shown that the exposure of human cancer cells to 50 and 385-Hz magnetic field resulted in the release of intracellular proteases that alter the integrity of cancer cell membranes (23). It has also demonstrated that the exposed mice breast tumor cells grow less into grossly visible tumors when compared with those unexposed to magnetic fields. However, the mice exposed at various times of exposure and intensities showed different tumor size and behavior such as rates of tumor growth and progression (24). Numerous studies have shown that exposure to electromagnetic fields leads to changes in cell differentiation and proliferation (25, 26), plasma membrane properties, and impaired calcium ion permeability, (27) or changes in the cytoskeletal organization (28). In the present study, the cytotoxic effects of continuous electromagnetic fields at 2 mT on AGS cells were demonstrated. There are conflicting results in the literature, and some studies have suggested that the electromagnetic field has proliferation-inducing effects (29, 30), while others have reported cytotoxic effects (31, 32). These contradictory results can be attributed to the intensity of the different magnetic fields as well as their state (continuous or discontinuous). In the present study, cytotoxic effects in the continuous magnetic field and stimulatory effects of proliferation in the discontinuous magnetic field were observed.
The present study showed that the electromagnetic field has a significant effect on the expression of the circ-CDR1as gene and this effect depended on the intensity of the magnetic field used and the cell type. CDR1as gene was differentially expressed in the exposed normal and tumor cells at the discontinuous and continuous magnetic field. On the other hand, exposure with up to the intensity of 0.5 mT of the discontinuous electromagnetic field resulted in the up-regulation of the circ-CDR1as gene in both tumor and normal cells. In contrast, down-regulation of circ-CDR1as gene in the normal and tumor cells was found at all intensity of the continuous electromagnetic field. The highest CDR1as expression belonged to 0.5 mT intensity of continuous and 2 mT intensity of discontinuous electromagnetic field in the normal and tumor cells, respectively. This finding showed that the continuous exposure to the tumor cell, as well as the discontinuous exposure to normal cells, resulted in up-regulation of the CDR1as gene. These findings showed that the changes in CDR1as gene expression induced by electromagnetic field may affect the survival rate of tumor and normal cells. The regulatory role of cellular processes by CDR1as is well known. Its mRNA plays a role by targeting microRNA-7 (miR-7) as a negative regulator (33, 34) and thus affects the expression of important genes. CDR1as have been shown to play an oncogenic role in cancers such as hepatocellular carcinoma cells (17), colorectal (35), breast (36), pancreatic cancer (37), and gastric (38) cancers. High expression of circ-CDR1as can be a sign of cancer (33) and in our study, increased expression of CDR1as in tumor cells was observed with increasing intensity of the continuous magnetic field, which may indicate the effect of continuous magnetic field on increasing tumorigenic activity. However, in the discontinuous magnetic field, it did not have much effect on the expression of circ-CDR1as, which can be attributed to the low time of continuous exposure. This has been confirmed in other studies (39). The activity of the PTEN gene in normal and tumor cells was also assayed at the various intensities of both continuous and discontinuous magnetic fields. We have found that the activity of the PTEN gene in the normal and tumor cells increased and decreased with increasing intensity of the discontinuous magnetic field, respectively. However, in the continuous magnetic field, the expression of this gene was decreased in both normal and tumor cells. It seems that the continuous magnetic field reduces the activity of this gene. This finding is not in accordance with cell viability results in which the continuous magnetic field resulted in a decrease in cell viability. PTEN is one of the tumor inhibitory genes that has been suggested that changes in this gene are associated with various cancers, including gastric cancer (11). The PTEN gene was first identified in brain, breast, and prostate cancers (9) and the expression of this gene has been shown to decrease in gastric cancer due to hypermethylation (40). In the current study, the low expression of this gene was also shown in tumor tissue compared with normal cells that are consistent with the results of the previous research mentioned above.
5.1. Conclusion
The results of the present study showed that the high density of continuous magnetic field has inhibitory effects on tumor cells and proliferative effects on normal cells. However, discontinues electromagnetic field showed no inhibitory effect on tumor cells. The up-regulation of the CDR1as and down-regulation of the PTEN genes in tumor cells were induced by a continuous electromagnetic field, which can reduce cell viability leading to tumorigenesis or cancer progression. In general, the effect of electromagnetic field on gastric cancer seems to depend on the kind of exposure, as well as a magnetic flux density and can be used for cancer therapeutic purposes. However, more research is needed on this subject.