1. Background
Human papillomavirus (HPV) causes genital warts, the most common sexually transmitted disease in the world. It is mainly transmitted through sexual intercourse. The importance of this virus is its role in increasing the risk of cervical cancer (1), the fourth leading cancer in women, which constitutes about 7% of mortalities due to cancer among women in 2018 (2). About 370 women died due to cervical cancer in our country in 2012, which is, according to predictions, an increase in all ages by 2035 (3). Cervical cancer mortality was 1.2 in Iran in 2012 (4). A review reported 42% of the ratio of deaths due to cervical cancer incidence (5).
Since 19704, HPV was introduced as the most important and best-known environmental cause of this cancer (6). Human papillomavirus with more than 100 genotypes and worldwide distribution is in the family of HPV viridian with the structure of a protein and double-stranded circular and uncoated genome (7).
This virus is transmitted sexually and molecular studies have shown that only its 15 types (16, 18, 31, 33, 34, 35, 39, 45, 52, 56, 58, 66, 67, 68, 82 types) called high-risk types and are carcinogenic (8).
Nonetheless, the global prevalence of HPV was reported 11.7% (9). A meta-analysis of healthy women suffering from cervical cancer in Iran reported HPV prevalence as 9.4% and 77.4% (10, 11).
Two preventive methods are recommended for appropriately controlling and fighting against cervical cancer; vaccination against HPV, and screenings after treatment of precancerous cancers (12). Serological diagnosis methods (Pap smear) of cervix cancer have some limitations resulting in the development of more sensitive approaches (13).
Continuous infection of certain types of HPVs such as 16 and 18 having E6 and E7 oncogenes, can cause cervical intraepithelial neoplasia (CIN) and cervical cancer (14, 15). Polymerase chain reaction can show the results very quickly; so, it is very helpful for detecting the DNA of HPV. Besides, it can be a good substitute for HPV testing. It is sensitive and can detect the nonviable virus. Recently, methods for diagnosing HPV lean on virus nucleic acid (16).
The genotypic distribution of HPV needs to be determined with detail as it is an important issue when it comes to public health and preparing vaccines since HPV types may be different in various areas (17, 18). Therefore, molecular techniques are very reliable for specifying the details and types of HPV considering the population data and regional background.
2. Objectives
This study aimed at reporting the frequency of HPV types among women in Sari in the north of Iran.
3. Methods
In this study, 50 women with genital warts, who had been referred to the Hospital of Sari, were studied. The participants are considered a random sample of the entire population for vaginal examination from October 2018 to April 2019.
Sampling was performed by a gynecologist, using sterile swabs of patients’ vaginal and cervical discharge under sterile conditions, followed by placing them in transport solution (5TPBS5T; 1 mL) and transferring to the molecular laboratory. The solution was homogenized, and the swabs were kept at a temperature of -20°С DNA from vaginal discharge that was done according to an extraction kit of DNG (Cinna Gen). Simultaneously with the multiplex PCR technique, evaluation of the extracted DNA was done regarding quantity (1.65 T < OD < 1.9) and quality using primer pairs PCO4 (5' CAACTTCATCCACGTTCACC-3') and PCO3 (5'-ACACAACTGTGTTCACTAGC-3') proliferating a part of the ß-globlobin gene. For the topping of 14 HPV types, namely 16, 18, 31, 33, 35, 39, 45, 52, 56, 58, 59, and 66, the specimens were assessed under three series of multiplex PCR according to specific kit primers of high-risk HPV (Sacace, Italy). In the first, second, and third series, PCR high-risk types, mediate-risk types, and low-risk types were evaluated, respectively. Finally, the photography and detection of PCR products under UV light after electrophoresis within 45 minutes on Agarose gel 2% and 3% including 0.5 - 1 mg in ml of ethidium bromide was done.
4. Results
The patients’ mean age was 38.3 ± 9.4 years (age range of 20 - 57). Based on personal expression, 90% of the sexual partners of the patients were 1 person. The mean and standard deviation of marriage duration was 17.08 ± 10.4 years, and in the majority of patients, the duration of marriage was under 10 years (40%).
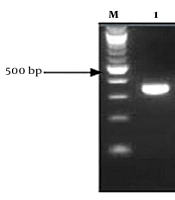
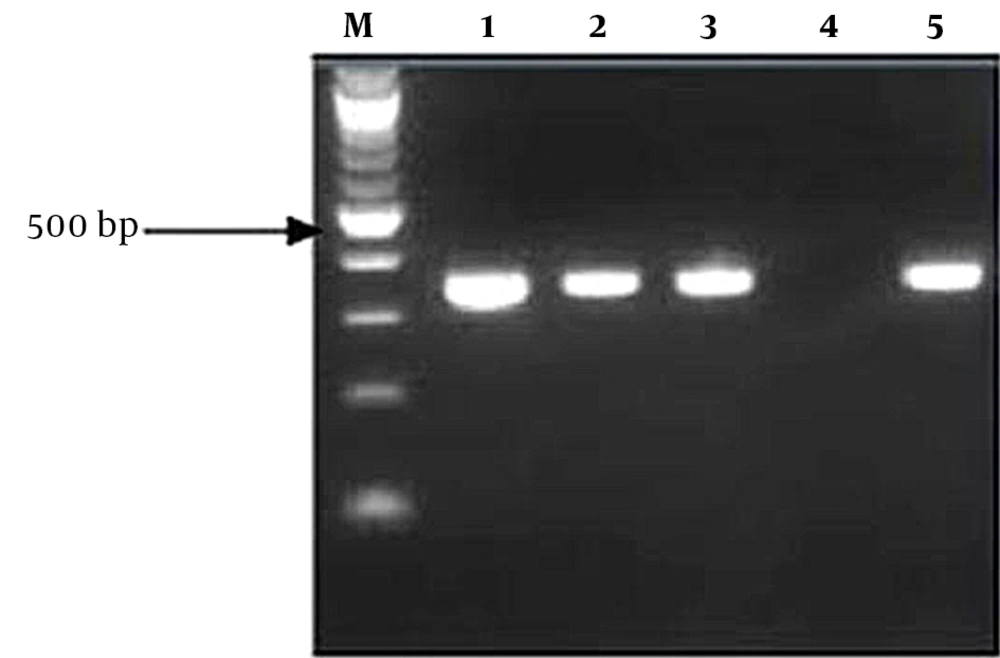
The presence of a 723 bp band after the PCR process about ß-globin displayed that the quality of all isolated DNA samples was good. The presence of various bands from 380 bp in the sample indicated the presence of HPV (Figure 1). Of 50 vaginal swab samples of patients with genital warts, 12 samples (24%) with 95% confidence intervals (37.3 - 13.8) were reported to be positive and 38 cases (76%) were negative regarding the HPV DNA presence (Table 1). The frequency of HPV types in women with genital warts included 5 patients (10.0%) with 2 high-risk serotypes as simultaneous serotypes 4% (16, 18), 4% (18, 35), 2% (66, 67), 1 patient (2%) HPV 33, 1 patient (2%) HPV 34, 1 patient (2%), 1 patient (2%) HPV 35, 2 patients (4%) HPV 66, 1 patient (2%) HPV 67, and 1 patient (2%) HPV 68 (Table 2).
| PCR | No. (%) | Frequency Confidence Interval (95%) | |
|---|---|---|---|
| Low Limit | Upper Limit | ||
| Pos | 12 (24.00) | 13.84 | 37.07 |
| Neg | 38 (76.00) | 62.93 | 86.16 |
Frequency Distribution of Human Papillomavirus Genotypes in Wart Patients According to PCR Test Results
| Genotypes’ of HPV | No. (%) | Abundance Confidence Interval (95%) | |
|---|---|---|---|
| Low Limit | Upper Limit | ||
| 16/18 | 2 (4.0) | 0.8 | 12.2 |
| 18/35 | 2 (4.0) | 0.8 | 12.2 |
| 33 | 1 (2.0) | 0.2 | 9.0 |
| 34 | 1 (2.0) | 0.2 | 9.0 |
| 35 | 1 (2.0) | 0.2 | 9.0 |
| 66 | 2 (4.0) | 0.8 | 12.2 |
| 66/67 | 1 (2.0) | 0.2 | 9.0 |
| 67 | 1 (2.0) | 0.2 | 9.0 |
| 68 | 1 (2.0) | 0.2 | 9.0 |
| Negative | 38 (76.0) | 62.9 | 86.2 |
Frequency Distribution of Human Papillomavirus Genotypes in Wart Patients According to Test Results
Based on Fisher's exact test and the results of Table 3, HPV genotypes in patients were significant in terms of genital wart (P < 0.01), vaginal secretion (P < 0.001), and multiplicity of sexual partners (P < 0.001). The chances of positive (OR) HPV genotypes in women with a history of warts, vaginal secretion, and the number of sexual partners of 2 persons were 26.6, 7.3, and 18.5 times. Based on our findings, the prevalence of HPV infection in women with genital warts, especially in Mazandaran, Sari, was 24%.
| Variables | HPV, No. (%) | P-Value | |
|---|---|---|---|
| Positive | Negative | ||
| Age | 0.837 | ||
| Under 30 year | 3 (27.3) | 8 (72.7) | |
| 31 - 40 year | 4 (20.0) | 16 (80.0) | |
| 40 years and up | 5 (26.3) | 14 (73.7) | |
| Duration of marriage | 0.843 | ||
| Less than 10 years | 5 (25.0) | 15 (75.0) | |
| 11 - 20 year | 4 (28.6) | 10 (71.4) | |
| 20 years and up | 3 (18.8) | 13 (81.3) | |
| History of genital warts | < 0.001; OR = 26.6 | ||
| Yes | 10 (62.5) | 6 (37.5) | |
| No | 2 (5.9) | 32 (94.1) | |
| Vaginal secretion | < 0.001; RR = 7.3 | ||
| Yes | 6 (100.0) | 0 (0.0) | |
| No | 6 (13.6) | 38 (86.4) | |
| The multiplicity of sexual partners | 0.009; OR = 18.5 | ||
| 1.00 | 8 (17.8) | 37 (82.2) | |
| 2.00 | 4 (80.0) | 1 (20.0) | |
| Number of children | 1.58 (0.79) | 1.68 (0.96) | 0.744 |
| Number of fertility | 2.00 (1.04) | 2.18 (1.37) | 0.672 |
Comparison of Frequency of Human Papillomavirus Genotypes in Patients Studied Based on Individual and Disease-Dependent Variables
5. Discussion
More than 450 000 cases of cervical cancer occur in the world annually. The number of related deaths has decreased in the last 30 years compared to the past. Each year, almost 200 000 deaths caused by cervical cancer occur; this rate is higher than 12 000 new cases of cervical cancer in developed countries annually. Of this rate, 4 000 deaths are associated with this disease (2). The majority of studies have demonstrated that several high-risk types of HPV affect the development of cervix cancer as they have been recognized in about 99% of cervix cancers in the world (19).
In our study, the prevalence of HPV was 20% by PCR. In the North of Iran (Mazandaran), the HPV presence in cervix cancer specimens was found to be 81.4% (20). Farjadian et al. assessed 101 patients with cervix carcinoma in Shiraz (Southern Iran), and 88 cases were HPV positive (87.1%) (21). Jabarpour Bonyadi et al. studied 75 recorded samples in formalin in Tabriz, and the prevalence of 62% was reported for HPV (22). Keyhani et al. assessed 100 people in Tehran and the prevalence of 73% was reported for HPV (23). Mahmoudi et al. in Yazd (southeastern Iran) declared the prevalence of 75% for HPV (24). Based on Bashi Zadeh Fakhar et al.’s study in Rasht, of 45 vaginal swap specimens of women with genital warts, 37 cases (82.2%) were positive by PCR (8). In another study, the distribution of HPV types was reported in Mashhad, Iran, where 74.1% of subjects were high-risk (25). Based on Mobini Kesheh and Keyvani’s study in 2019, the total HPV prevalence was 49.5% (26). Human papillomavirus is a DNA virus (27).
A huge amount of studies have been performed on HPVs' effect and risk in causing cervical cancer (28). According to Allameh et al., 90.8% of HPV types were detected in cervical cytology (29). Another study reported the presence of HPV DNA in 30.7% of cervical carcinoma cases (30). A meta-analysis found a 79% HPV overall prevalence in high-grade squamous intraepithelial lesions, and 62% in low-grade intraepithelial lesions. The virus was noticed in 9% of the normal population, 5.5%, and 13.6% in the south and north of Iran, respectively (10). Khodakarami et al. in a research on HPV demonstrated that HPV was observed in 7.8% of the general population, lower compared to other countries (31). In Fars, 87.1% of patients suffering from cervical cancer had HPV based on their DNA (21). However, no association was observed between HPV type and tumor histology (32). A systematic review of studies performed in Iran and a survey on the national cancer registry reported having HPV in 76% of women who had cervical cancer. In a study, the commonest types of the virus included HPV16 (56%), HPV18 (15%), and HPV 31 (10%), 7% of the population included women (5). A study found HPV DNA in 5.5% of healthy females, and high-risk types were observed in 2% of the women (32).
Our results show 6 common types of HPV types 33, 34, 35, 66, 67, 68, and concurrent genotypes such as 16/18, 18/35, and 66/67. Genotypes 16 and 18 had more frequency in our study. In a multicenter study conducted in 7 countries in 2002, HPV16 was the most prevalent type between 43.9% and 72.4% (33). According to the results of a study compatible with our findings, HPV16 (in 50% of cases) and HPV18 were the most frequent virus types in women, who had cervical carcinoma (12%) (34). Research in Yazd, Iran (24) scrutinized HPV genotypes in cervical cancer, suggesting the high prevalence of HPV16 (70%) and HPV18 (16.7%) (35). These findings confirm those of our research. According to a comprehensive genotyping on 20 000 Pap smear specimens in Kerman, Iran, HPV16 and 18 had the highest prevalence (36). In Yazd and Mazandaran , HPV16 was the commonest type in cancer patients (24). Human papillomavirus 16 was the commonest type in Tabriz according to Jabarpour Bonyadi et al. with a frequency of 64.5% (22). In Guilan, the frequency of genotype 16 was reported 10.8% (8). Based on Mobini Kesheh and Keyvani, genotypes 16 and 18 had more frequency (26).
Based on our study, HPV genotypes in patients were significant in terms of genital wart, vaginal secretion, and multiplicity of sexual partners. According to epidemiological studies, the young age of the first intercourse, the number of intercourse, multiple sexual partners, and non-observance of genital hygiene are the most important risk factors for cervical cancer (37). Other factors include the number of pregnancies, the emergence of the first pregnancy at an early age and before the age of 18, sexual contact with high-risk men (men who have sexual contact with multiple women), long-term use of oral contraceptive pills, smoking, low social and economic status, lack of genital hygiene and immunoseparary medications (38). Such difference in the pattern may because of variations in evaluated cases i.e. cases in Mazandaran and Tehran follow a global pattern, while southern areas of Iran have a different pattern (8, 26).
Human papillomavirus genotype varies in diverse areas. Thus, the genotypic distribution of any population should be specified before making health care decisions and conducting vaccination plans (12, 39). The findings can be applied to develop medical approaches for the simultaneous targeting of the virus multiple and specific types (40).
In this study, 6 patients, despite having genital warts, the PCR result was negative for the presence of HPV. In the study of Hajibagheri et al. which was conducted on 50 women, in 22 of them, the result of the PCR test was negative, despite the lesions inside and outside the vagina (41). However, more studies are needed in this field to obtain more documentation.
Due to the high cost of molecular testing and the lack of easy access to women with genital warts, the population under our study was considered to be 50 women. It is clear that more research is needed. Further studies should also be performed on a larger community of women with genital warts in this geographical area.