1. Background
Melanoma is a malignant tumor of the skin that is originated from melanocyte cells. Cells become proliferative, dysplastic, expand from epidermis to dermis, and can quickly metastasize to other organs (1). The prevalence of melanoma varies in different geographical regions and between races. The global incidence of melanoma has increased rapidly in recent decades and its prevalence is doubled every 10 to 20 years (2, 3).
Human skin melanoma is one of the most severe skin cancers and has a high prevalence and mortality rate. Currently, in the United States, melanoma is the 5th most common cancer in men and the 6th most common cancer in women (4), which is estimated to be even higher due to the lack of proper diagnostic criteria (5).
Melanoma has various risk factors including exposure to sunshine, genetic and epigenetic factors, immunosuppression, multiple and abnormal nevi, as well as occupational and nutritional factors (6-9). The most potent risk factor is exposure to type B ultraviolet radiation (UVB), while type A (UVA) such as tanning and psoralen plus ultraviolet A (PUVA) therapy can increase the chance of melanoma (10).
Many efforts have been made to achieve effective therapies for the disease. Common therapies are surgery, chemotherapy, hormone therapy, radiation therapy, gene therapy, and immunotherapy. Treatment for local lesions in the early stage is surgery, while in advanced cases, response to treatment is weak because they are metastatic and resistant to common medications such as single-drug chemotherapy regimens and radiotherapy, suggesting novel, effective, and low-risk therapies (11).
The use of medicinal plants for cancer treatment has always been an option for researchers (12, 13). Iranian scientists have provided different natural therapies for modulating cancers in traditional Persian medicine (14). Iris germanica L., known as “Irsa” in Persian medicine, is a herb and its rhizome has been used for the treatment of a skin cancer similar to melanoma (15-17).
The plant is a member of the Iridaceae family and has many varieties. This plant is popular in many parts of the world including Iran (18). The rhizome of Iris germanica is a rich source of flavonoids such as irigenin, nigricin, irilone, and irisolidone (19-21). Benzene derivatives have also been obtained from the methanolic extract of the Irsa rhizomes (22). The fresh rhizome of Irsa contains triterpenoids called “iridals”, which are the precursor of iron. Irones are also extracted from the dried rhizome of Irsa and used for medical and perfumery purposes (23). In addition to these compounds, various types of sterols including sitosterol and stigmasterol have been extracted from the Irsa rhizomes (24). Furthermore, antioxidant, anti-inflammatory, anti-bacterial, and anti-ulcer properties of Irsa compounds have been investigated in several studies (18, 21, 25).
2. Objectives
Previous studies have demonstrated anti-mutagenic, anti-proliferative, and cytotoxic effects of the Irsa extract in different cancer cell lines (16, 25-27), while no studies have been performed so far on the impact of the whole extract of Irsa on A375 (human skin melanoma) cancer cell line. In this study with the inspiration of traditional Persian medicine, the effect of Irsa extract on proliferation and apoptosis of the A375 melanoma (cancer) cell line was investigated for the first time and compared with the AGO-1522 (normal human fibroblast) cell line.
3. Methods
3.1. Preparation of Plant Sample
The complete plant of Iris germanica with blue flowers and the Herbariums registration number of S-A1037-131 in the Plant Extract Bank was obtained from Karaj, Iran. The plant is also called "Susan Assemanjooni" according to the traditional and allopathic medicine references and used after confirmation by the Herbal Medicines Department of the Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The rhizome was separated from the rest of the plant, dried in shadow, chopped, and kept in a cold place until extraction.
3.2. Extraction and Condensation
Two hundred grams of the chopped rhizome was added into a mixture of water (480 mL) and (120 mL ethanol) for 72 hours. Extraction of the chopped rhizome by ethanol has the lowest damage to compounds such as alkaloids, saponins, carbohydrates, tannins, and flavonoids (28, 29). Next, the extract was filtered with a Whatman filter paper No. 1, incubated in a 70°C water bath for 12 hours, concentrated, and dried. The extract was stored in a refrigerator at 4°C for subsequent molecular experiments.
3.3. A375 and AGO-1522 Cell Lines
The A375 human melanoma cell line (C10141 IBRC, Iran) is originated from a 54-year-old woman with malignant melanoma in 1973.
The AGO-1522 cell line, which is a normal human fibroblast cell line, was used as a healthy control cell line. The cells were taken from the skin of a 3-day-old (male) newborn.
3.4. Cell Culture
A375 and AGO-1522 cell lines were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS).
Cells (104 cells) were cultured in each well of a 96-well plate with 100 μL of culture medium. In the next step, the plant extract was dissolved in dimethyl sulfoxide (DMSO) and cells were treated with different concentrations (0.031, 0.0625, 0.125, 0.25, 0.5, and 1 mg/mL). Then, cells were incubated in an incubator with 37°C, 5% CO2, and humidified atmosphere for 48 hours.
In addition to the Irsa extract, the A375 and AGO-1522 cell lines were treated with 100, 250, 500, 750, and 1000 μM concentrations of dacarbazine (Sigma Chemical Co., St. Louis, MO, USA) as a chemotherapeutic agent and positive control. In previous studies, a range of 100 to 1000 μM was used for cytotoxicity (30, 31).
3.5. MTT Assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was prepared at a concentration of 5 mg in a serum-free RPMI medium. The supernatant of cell lines was removed and incubated with the different concentrations of the Iris extract in the 96-well plate. Then, the MTT solution was diluted with serum-free RPMI with a 1:1 ratio, and 100 μL of the obtained solution was added to each well of the plate and incubated at 37°C for 3 hours. Then, the solution was removed, and 100 μL of 0.04 HCl-containing acidic isopropanol was added. After the appearance of the violet color of formazan crystals, the plates were read at 570 nm wavelength, using an enzyme-linked immunosorbent assay (ELISA) reader (BioTek ELISA reader, USA).
3.6. Flow Cytometry Analysis
Flow cytometry was performed to evaluate the viability of cells treated with 0.015, 0.0625, and 0.031 mg/mL concentrations of the Irsa extract. 1000 μM dacarbazine and 0.5% DMSO were used as positive and negative controls, respectively. Flow cytometry was carried out according to the BD kit instruction. Briefly, the supernatants of wells treated with the Irsa extract and dacarbazine were removed, and cells were isolated, using trypsin. Then, the cell suspension was divided into four 1.5 mL microtubes and centrifuged at 1500 rpm for 5 minutes. After removing the supernatant, 100 μL binding buffer was added to the cells. Five microliter Annexin V was added to 2 microtubes and incubated for 15 minutes; then, 5 μL propidium iodide (PI) was added to one of the two microtubes. Five microliter PI was added to one of the two primary microtubes that only had the binding buffer, and the other microtube remained intact. All microtubes were incubated for 2 minutes and, then, read by flow cytometry (BD FACSCalibur flow cytometer, USA). In addition to dacarbazine and Irsa extract, 0.5% DMSO was used as a negative control.
3.7. Statistical Analysis
Each MTT assay was performed with 8 repetitions. The growth curve was also obtained, using the equation of the concentration regression line. The cell growth rate was calculated, using the below formula:
Finally, IC50 (the drug concentration that destroys 50% of the cells) was obtained by using the plotted curve. Indeed, IC50 was used to quantify the measure that indicates how much of the Irsa extract is needed to apoptosis the cells by 50%. The effects of different levels of dacarbazine and Irsa extract on A375 and AGO-1522 cell lines were compared with one-way ANOVA, using SPSS release 25.0 (SPSS Inc., Chicago, IL, USA). P-values of < 0.05 were considered significant.
4. Results
4.1. MTT Assay on A375 Melanoma and AGO-1522 Control Cell Lines
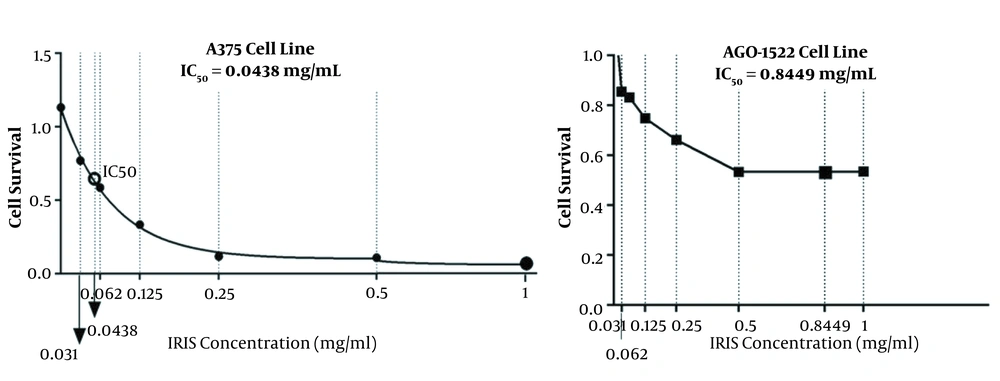
According to the MTT assay, control and melanoma cancer cell lines had different sensitivities to the various concentrations of dacarbazine and Iris extract. The viability of the cells was evaluated, using MTT. The maximum inhibitory concentration (IC50) of the Irsa extract for A375 melanoma cells was 0.0438 mg/mL after 48 hours of treatment. At the mentioned concentration, 50% of the cells in the plate lost their proliferation and viability. In addition, IC50 of the Irsa extract for the AGO-1522 control cell line was 0.8449 mg/mL and about 50% of the cultured cells lost their viability (Figure 1 and Table 1). Therefore, melanoma cancer cells were destroyed at 0.0438 mg/mL of Irsa extract, while the viability of control cells was not affected.
| Cell Line | Treatment | IC50 (mg/mL) |
|---|---|---|
| AGO-1522 | Iris extract | 0.8449 |
| A375 | 0.0438 |
a MTT assay of 1000 μM of dacarbazine on the cell lines showed the highest toxicity. Therefore, this concentration was used as the positive control.
4.2. Flow Cytometry of Irsa Extract on A375 Melanoma and AGO-1522 Control Cell Lines
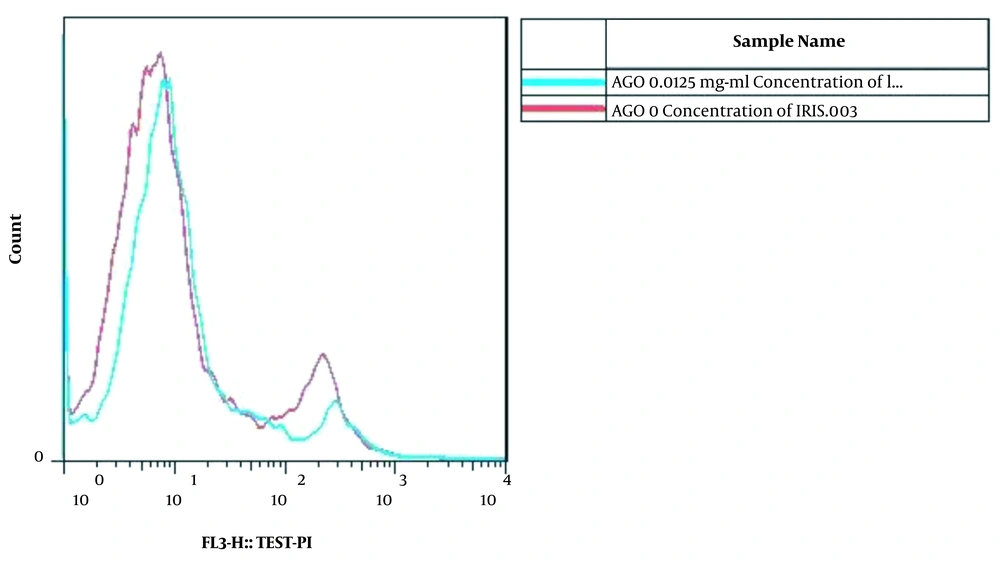
The viability of the AGO-1522 cells in 0.125 mg/mL of Irsa extract was similar to the negative control. Therefore, the 0.125 mg/mL concentration of Irsa extract was the most suitable recommended concentration with a low cytotoxic effect on the AGO-1522 cells (Figure 2). However, 0.25 mg/mL and 0.5 mg/mL concentrations of the extract led to 77.88% and 70.7% of apoptosis in the AGO-1522 cell line, respectively.
Flow cytometric analysis and comparison between the 0.125 mg/mL with zero concentration (negative control) of the Irsa extract in AGO-1522 cells. At the 0.125 mg/mL of Irsa extract, the viability of the cells was similar to the negative control. Therefore, the 0.125 mg/mL of Irsa extract is the most suitable recommended concentration that has a lower cytotoxic effect on AGO-1522 cells.
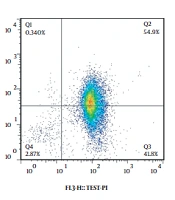
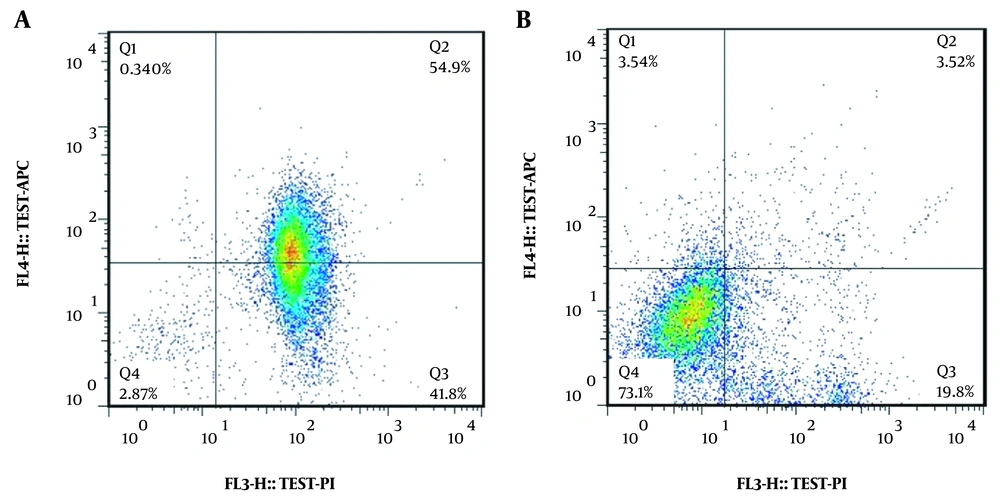
According to our results, 0.125 mg/mL of Irsa extract lead to 96.7% apoptosis in the A375 melanoma cell line, whereas the same concentration had the most viability and the least apoptosis rate (24.33%) in the AGO-1522 cell line (Figure 3 and Table 2).
| Category | Treated Cells | Non-treated (DMSO) (Control -) | Dacarbazine (Control-+) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.031 mg/mL | 0.0625 mg/mL | 0.125 mg/mL | 0.125 mg/mL | 0.25 mg/mL | 0.5 mg/mL | |||||
| Cell Type | A375 | A375 | A375 | AGO-1522 | AGO-1522 | AGO-1522 | A375 | AGO-1522 | A375 | AGO-1522 |
| Apoptotic Cells | 5.51 | 48.45 | 55.24 | 8.76 | 11.06 | 48.9 | 4.26 | 8.72 | 91.2 | 77.9 |
| Live Cells | 83.9 | 3.11 | 2.87 | 72.1 | 19.9 | 18 | 84.8 | 71.9 | 8.53 | 18.4 |
| Necrosis | 10.6 | 48.4 | 41.8 | 19.2 | 69 | 33.1 | 10.9 | 18.7 | 0.230 | 3.75 |
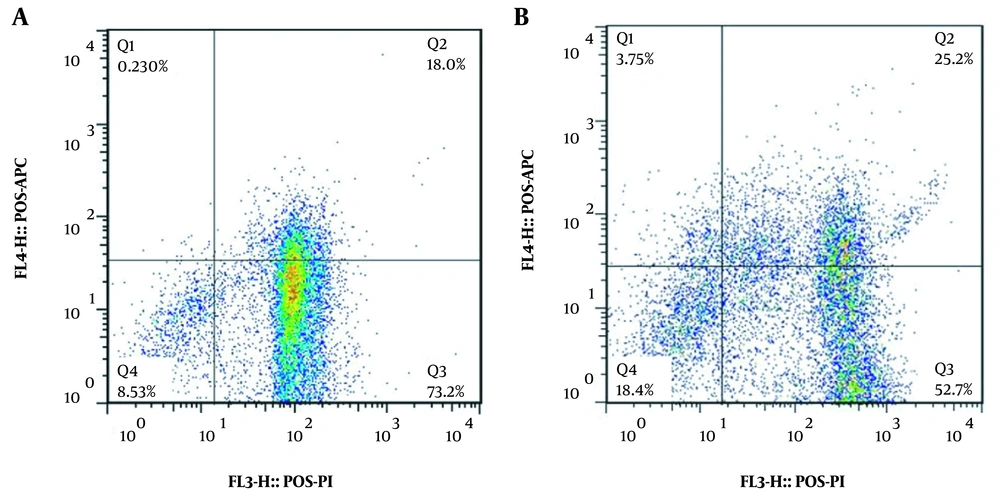
Besides, the viability of the AGO-1522 control cells and the A375 melanoma cells treated with 1000 μM of dacarbazine were 18.4% and 8.53%, respectively. Moreover, the viability of the AGO-1522 control cell line and the A375 melanoma cell line treated with the 0.125 mg/mL of the Irsa extract was 72.1% and 2.87%, respectively (Figure 4).
4.3. Statistical Findings
Statistical analysis with ANOVA test showed a significant difference in growth inhibition of cancer cells treated with different levels of Iris extract (P < 0.001). In addition, there was a significant difference in growth inhibition of the AGO-1522 cell line treated with various levels of Irsa extract (P < 0.001).
Moreover, a significant difference was observed between the inhibitory effects of Irsa extract on induction of apoptosis in the A375 cell line compared to the AGO-1522 cell line (P < 0.001).
5. Discussion
Melanoma is one of the most common and malignant forms of skin cancer that originates from melanocyte cells. Melanocytes are pigment-including skin cells that produce pigment in response to irritations such as UV radiation. These cells are responsible for the color of skin, eyes, and hair. Environmental and genetic factors are involved in their development (32).
According to the World Health Organization (WHO), the incidence of melanoma is rapidly increasing in comparison to other skin cancers (33). The conventional treatments for melanoma including immunotherapy, chemotherapy, radiotherapy, and surgery did not show promising results and had their limitations (34). Recently, few effective treatments have been developed including immunotherapy with interferon (IFN) alfa-2b, interleukin (IL)-2, anti-CTLA-4, ipilimumab, and BRAF and MEK inhibitors. These treatments had many side effects, long-term responses, and were cost-effective (35-37). Due to these problems, alternative therapies are highly welcomed in this field (38). In the present study, Iris germanica L., known as Irsa, was selected to achieve an effective and less toxic treatment for melanoma.
In 2016, scientists at the Applied Medical Center of Korea showed that the ethanolic extract of Gardenia jasminoides Ellis roasted fruit inhibited migration of metastatic melanoma pulmonary tumor cells and reduced angiogenesis (39). In another study, herbal medicine from the ginseng family (Ginsenoside Rg3) was introduced to the scientific community as a unique product for the treatment of melanoma. This herbal medicine was able to inhibit mediators involved in tumor growth such as fucosyltransferase IV (FUT4) and induced apoptosis in a melanoma cell line (40). Furthermore, Chinese scientists identified the molecular mechanisms implicated in cell cytotoxicity of the Physalin A on the A375-S2 human melanoma cell line. This compound is an active withanolide that is derived from the physalis alkekengi L. and the Makino shrub called Jin Deng Long in Chinese traditional herbal medicine. The cytotoxic effect of the Jin Deng Long on the melanoma cell line has been investigated in previous studies (41). In recent years, Chinese researchers have also investigated the cytotoxic effect and anti-tumor mechanism of Chinese traditional herbs and their derivatives on melanoma and pulmonary metastatic melanoma (42-45).
None of the studies have investigated the cytotoxicity effect of the herbs extract on the A375 cell line; therefore, no comparison can be performed with the current study. However, the cytotoxic effects of certain compounds of the Irsa extract on different cancer cell lines other than melanoma have been investigated in previous studies.
The protective effects of isoflavonoids of the Irsa rhizome extract on the inflammatory condition of the Hepa-1c1c7 cell line were evaluated. It was revealed that the compounds present in the Irsa extract could inhibit the activity of cytochrome P450 1A. In addition, isoflavone 2, 3, and 5 can induce NAD(P)H quinone reductase activity. The effects of Irsa on cancer cells are exerted through the activation of anti-inflammatory mechanisms (46). The anticancer effects of isoflavonoids of the Irsa extract on the EAC cancer cell line have also been studied, and the results showed that this product could inhibit the proliferation of the cancer cells (25).
In another study, the therapeutic effects of lipid compounds of the Irsa extract on the Hela cancer cell line were investigated. The study showed that the Irsa extract could inhibit migration of the cells and maintain cells in the G1 phase of the cell cycle. The Irsa extract prevents the migration of cancer cells by increasing adhesion molecules and producing more actin fibers (47).
The superiority of triterpenoids of the Irsa extract to doxorubicin was demonstrated in the A2780 and K562 cancer cell lines. Although these cell lines are resistant to a large number of chemotherapy drugs, they respond properly and showed cytotoxicity against the Irsa extract (48).
So far, no studies have been carried out to evaluate the Irsa extract therapeutic effects on the A375 melanoma cell line. Our study was performed for the first time to study the effects of the total Irsa extract on proliferation and apoptosis in the A375 melanoma cell line compared these effects with the AGO-1522 as a control cell line.
According to the present study, 0.0438 mg/mL of the Irsa extract destroys 50% of the A375 melanoma cell line, while no significant destructive effect on proliferation and viability of the AGO-1522 cell line was not observed.
Based on the flow cytometry results, the Irsa extract, similar to dacarbazine, lead to apoptosis in the melanoma cancer cells. Nonetheless, the Irsa extract, unlike dacarbazine, had no cytotoxic effect on normal cells. In addition, flow cytometry results showed that 0.125 mg/mL of Irsa extract had the most cytotoxic effect on the A375 cell line and the highest rate of apoptotic and necrotized cells were observed. According to the results, the best-recommended concentration for A375 melanoma and AGO-1522 control cell lines is 0.125 mg/mL of the Irsa extract. This concentration not only had the highest cytotoxic effects on the A375 melanoma cell line but also had the lowest cytotoxic effects on the AGO-1522 control cell line.
5.1. Conclusions
We evaluated cytotoxic effects and apoptosis induction of the Iris germanica L. total extract on the human skin melanoma cell line (A375) for the first time. Our results showed that 0.125 mg/mL of the Irsa extract significantly increase apoptosis in melanoma cancer cells, while it was entirely safe for the normal cell lines. Therefore, this compound can be considered a novel, side-effect-free treatment for human skin melanoma.