1. Background
Breast cancer is the most common malignancy in women worldwide (1). Globally, the incidence rate of breast cancer is high, identifying 2 million new cases in 2018. In Iran, the prevalence of breast cancer is also high, with an annual incidence rate of 33.21 per 100,000 women and the mortality rate associated with this cancer is 14.2 per 100,000 women, with a mean age of 49.84 years old (2). Breast cancer, the most prevalent type of neoplasm among Iranian women, is often diagnosed when it reaches the advanced or metastatic stages. Therefore, the quality of life in these patients is profoundly disrupted due to the physical and psychological burden associated with the disease; however, it could be restored to some extent via prognostic factors. The rate of lymph node metastasis and the number of positive lymph nodes are prognostically important, particularly in some subtypes of breast carcinoma including invasive micropapillary cancer (IMPC). IMPC is a rare and aggressive subtype of breast cancer, initially described in 1980 (3), and histologically distinguished from other subtypes. Micropapillary structures are clusters of tumor cells settled in the clear vascular-free space derived from polarity inversion of tumor cells (4). Beside pure IMPC, which is a distinct subtype, mixed IMPC is present in which the micropapillary component is observed in association with other subtypes of breast cancer, particularly invasive ductal carcinoma (IDC) (4). Tumors with any proportion of micropapillary components are aggressive and associated with a high rate of lymph node involvement and poor prognosis and outcome. Lymph node dissemination is common in IMPC due to its angioinvasive characteristics. The mechanisms by which a tumor can metastasize are various and generally comprised of several steps. Following the loss of connections, tumor cells can penetrate the vessel wall, enter new tissues, and perform angiogenesis. Numerous studies have attempted to clarify the main cause of increased lymph node metastasis in IMPC, but the answer remains unclear. However, previous studies have shown that heat shock protein 27 (Hsp27) and Leukocyte 1 cell adhesion molecule (L1CAM) can explain pathogenic features of IMPC and the high rate of lymph node involvement observed in this cancer (5, 6). Furthermore, the level of CD24 expression in IMPC should be considered since the role of CD24 in the metastasis of other cancers has been previously confirmed (7). Higher expression of CD24 in IMPC compared to invasive ductal carcinoma, not otherwise specified (IDC-NOS), was reported in a study by Reyal et al. This can explain the higher rate of invasion observed in IMPC and also make CD24 a potential therapeutic target (8).
2. Objectives
The aim of the current study was to evaluate the rate of lymph node metastasis and its association with pathological variables such as patient's age, tumor size, grade and focality, lymphovascular invasion, and proportion of micropapillary component in invasive micropapillary cancer and compare it with invasive ductal carcinoma, not otherwise specified (IDC-NOS), used as control.
3. Methods
The study was conducted on 124 patients with invasive breast carcinoma who were referred to Shohada-e-Tajrish Hospital (Tehran, Iran) from 2009 to 2014. The Ethics Committee of Shahid Beheshti University of Medical Sciences approved the study, and informed consent was obtained from the patients. The patients’ information has been kept confidential and was accessible only by the main researchers of the study.
Pathology reports and paraffin blocks of patients were retrieved from the Pathology Department. Patients were classified into 2 groups including cases with non-specific invasive ductal carcinoma and those with invasive micropapillary breast cancer who had undergone an excisional biopsy and a breast-removing surgery such as simple (SM), radical (RM), and modified radical mastectomy (MRM). To be included in the study, the patient’s tumors should be removed, and axillary lymph node dissection should also be performed. Cases without axillary lymph node excision and those with core needle biopsy were excluded from the study. For all patients from both groups, tumor and axillary lymph nodes slides were carefully examined separately by two pathologists. The histologic parameters evaluated in this study were; the histological grade based on Nottingham combined histologic grade, the presence or absence of vascular-lymphatic invasion, and lymph node involvement. For patients with invasive micropapillary breast cancer, the proportion of micropapillary components was also assessed. Information such as the patient’s age, tumor size, and focality was retrieved from the patient’s medical records.
Data analysis was done using SPSS software version 24. Statistical tests such as the independent t-test, chi-square, and Fisher tests were used in this study. Data less than 0.05 were considered significant.
4. Results
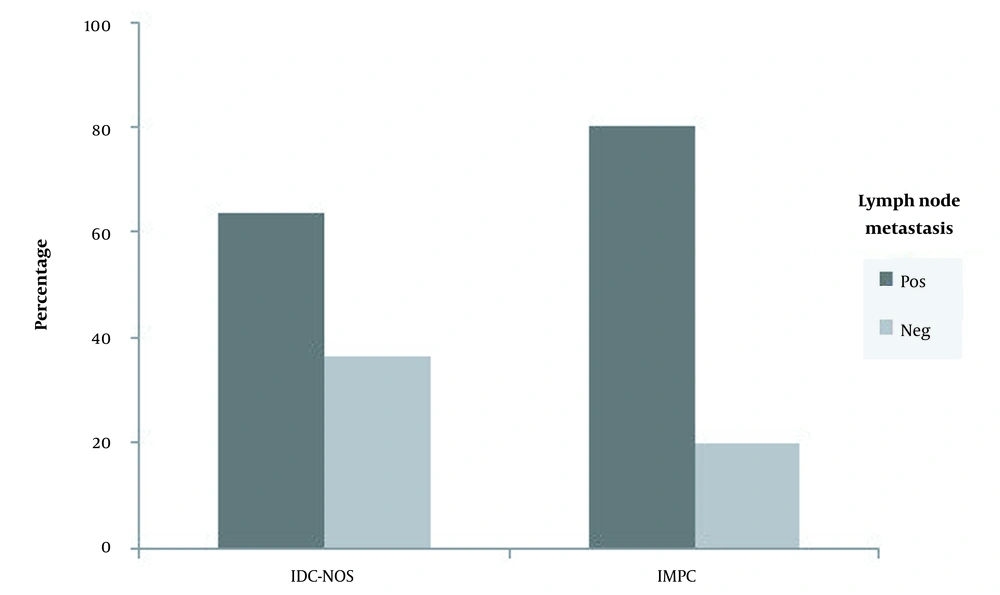
A total of 1163 patients with invasive ductal carcinoma were histopathologically ascertained in the Pathology Department of Shohada-e-Tajrish Hospital from 2009 to 2014. Of these, 61 patients with IMPC (mixed with IDC) and 63 cases with invasive ductal carcinoma, not otherwise specified (IDC-NOS), could finally meet the inclusion criteria and enter the study. Note that lymph node involvement was found in 80.3% and 63.5% of patients with IMPC and IDC-NOS, respectively. Note that the above-mentioned difference was statistically significant (Figure 1).
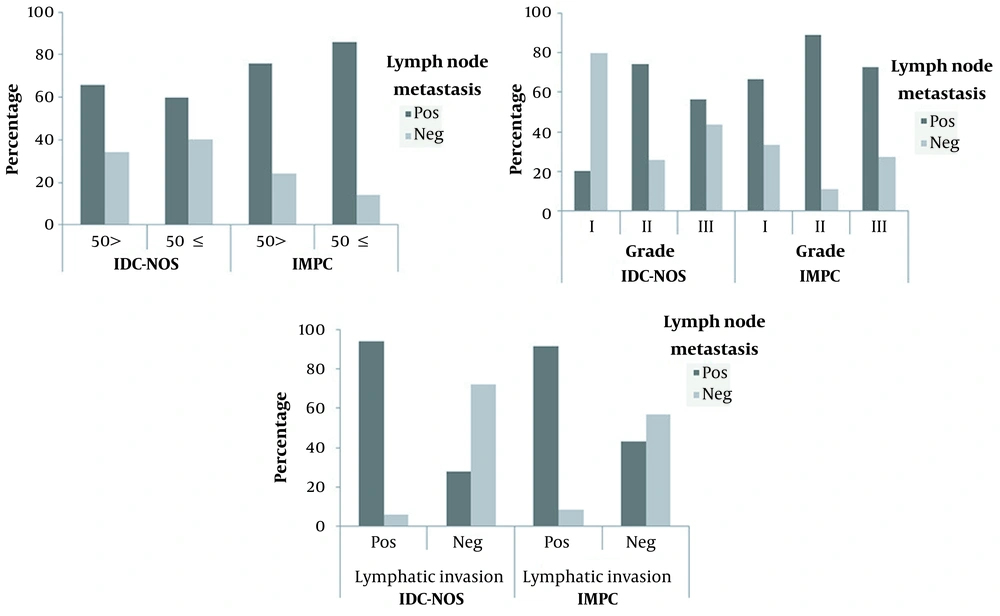
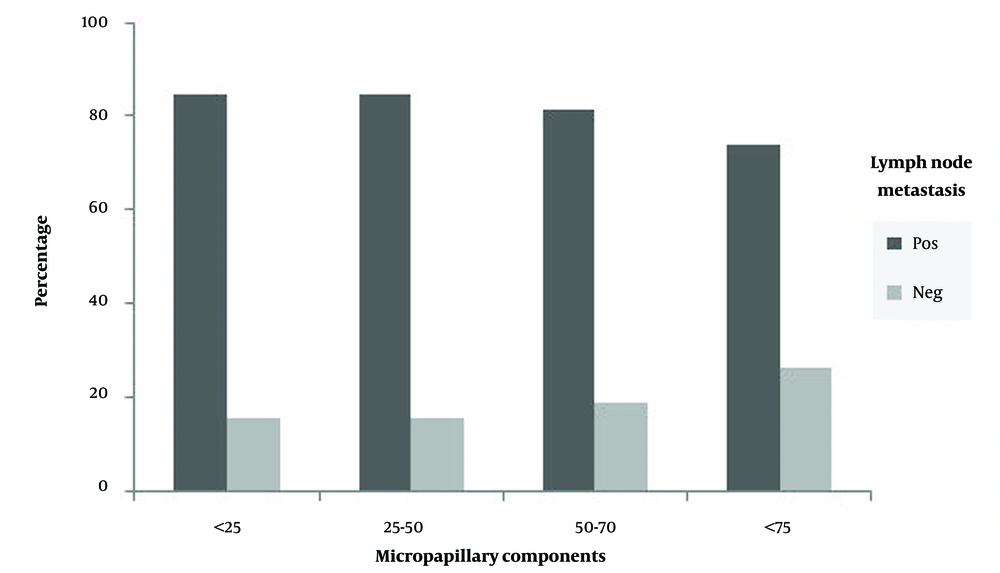
In both IMPC and IDC-NOS groups, patients were distributed into two age groups, including patients up to 50 years old, and patients aged 50 years and older. In the IDC-NOS group, 60% of patients aged 50 and over revealed nodal metastasis, while lymph node metastasis was observed in 65.8% of patients under 50 years of age. In the IMPC group, patients aged ≥ 50 years exhibited a higher rate of lymph node metastasis compared to younger patients, with a rate of 85.7% and 75.8%, respectively. According to the results derived from statistical analysis, no significant correlation was found between nodal metastasis and the patient’s age in both groups (Figure 2A). Tumor size in both IMPC and IDC-NOS groups ranged from less than 2 cm to greater than 5 cm. No significant relevance was observed between nodal metastasis and tumor size. Also, none of the unifocal and multifocal tumors showed a significant correlation with nodal metastasis in both groups. All patients in each group were categorized into 3 groups (I, II, and III) concerning histological grades. Of these, most patients presented with grades II and III tumors. In the IMPC group, lymph node metastasis was found in 66.7%, 89.3%, and 72.7% of patients with grades I, II, and III, respectively. In the IDC-NOS group, 74.3% of patients with grades II and 56.5% of patients with grade III showed lymph node involvement, whereas 20% of patients with grades I were lymph node-positive. Therefore, no obvious relationship was observed between nodal involvement and tumor histological grades in patients with IMPC, whereas a direct connection was detected between lymph node metastasis and tumor grades in the IDC-NOS group (Figure 2B). In the IMPC group, 94.1% of patients with lymphovascular invasion exhibited lymph node involvement. Likewise, in the IDC-NOS group, lymph nodes metastasis was seen in 94.1% of patients with lymphovascular invasion. Therefore, in both IMPC and IDC-NOS groups, patients with positive lymphovascular invasion showed a significantly higher rate of lymph node dissemination (Figure 2C). In the IMPC group, patients were divided into 4 groups according to the percentage of microcapillary components (< 25%, 25 - 50%, 50 - 75%, and > 75%). Patients with any percentage of micropapillary components showed a high degree of lymph node involvement. Therefore, no statistically meaningful link was noticed between lymph node metastasis and the percentage of micropapillary components (Figure 3).
The relationship between patient’s age (A), tumor grades (B), and lymphatic invasion (C) and lymph nodes metastasis in patients with invasive ductal carcinoma, not otherwise specified (IDC-NOS) and invasive micropapillary breast cancer (IMPC). A: No significant correlation was found between lymph nodes metastasis and the patient’s age in both IDC-NOS and IMPC. B: No significant relationship was observed between lymph node metastasis and tumor grades in IMPC, while this correlation was statistically significant in patients with higher tumor grades of IDC-NOS. C: In both types of cancer, patients with lymphatic invasion showed a significantly higher rate of lymph node metastasis.
5. Discussion
In the present study, we assessed the rate of lymph node metastasis and its possible relationship with pathological variables such as age, tumor size, grade and focality, lymphovascular invasion, and proportion of micropapillary component in invasive micropapillary cancer (mixed entity) compared to invasive ductal carcinoma, not otherwise specified (IDC-NOS). Metastasis to lymph nodes can play an important role as a prognostic factor, particularly in several types of breast cancers including invasive micropapillary cancers (9-11). The number of nodal involved can also have prognostic value (12-14). In the current study, we noticed a higher rate of metastasis to lymph nodes in patients with IMPC compared to IDC-NOS. Compared to our study in which 80.3% of patients with IMPC had nodal involvement, a lower rate (52.9%) of metastasis to lymph nodes was reported by Chen et al. in a study involving 624 patients with micropapillary breast cancer (15). In the above-mentioned study, they observed that the survival rate had reduced in cases with more than 4 involved positive lymph nodes. In another study conducted in 2014 by the same group, they assessed the rate of lymphatic metastasis in 636 patients with IMPC compared to 297735 patients with IDC-NOS (16). They reported a rate of 52.9% and 34.6% of lymph node metastasis in patients with IMPC and IDC-NOS, respectively, which is much lower compared to our study. Also, they found that patient’s prognosis was inversely associated with tumor size and the number of positive lymph nodes. However, we did not find such a correlation between a patient’s prognosis and the tumor size.
A study by Yu et al., included 72 patients with IMPC and 144 cases of invasive ductal carcinoma, they revealed that lymph node metastasis was more common in IMPC cases, and these patients also showed a higher rate of non-response to treatment (17). In another study by Guo et al., comprised of 51 patients with micropapillary breast cancer and 1056 cases of invasive ductal carcinoma, they discovered a significantly higher incidence of both lymph node metastasis and the number of positive lymph nodes in cases with IMPC compared to IDC (18). These findings are consistent with the results of the current study. In a cross-sectional study published in 2015 by Cui et al., a similar rate (80%) of lymph node involvement was found in patients with IMPC (19). Paterakos et al. reported a decreased survival rate in patients with IMPC compared to IDC patients (20). In this study, the evaluation of the prognosis of patients was not possible due to some limitations. However, it could be the subject of future research.
5.1. Conclusions
A high rate of lymph node metastasis irrelevant to tumor grade in patients with invasive micropapillary cancer (IMPC) can be used as an important prognostic factor to distinguish this subtype of breast cancer from IDC-NOS and improve therapeutic outcomes in these patients by taking appropriate interventions.