1. Introduction
New coronavirus or severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) emerged in Wuhan, China, at the end of 2019 (1) and rapidly spread throughout the world. World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) a pandemic on 11th 2020 (2). The disease caused by SARS-CoV-2 named COVID-19 (3).
SARS-CoV-2 is an RNA virus with a 30 kb size (4). In most cases, the virus transmission occurs from human to human via respiratory droplets and contact (3). The incubation period varies from 2 to 14 days (2).
The severity of the disease greatly varies from patient to patient. Some patients have no symptoms and most of the patients have the mild disease (5). Actually, 80% of patients have mild to moderate symptoms [13.8% are infected with the severe form (dyspnea, respiratory frequency ≥ 30/minute, blood oxygen saturation ≤ 93%, PaO2/FiO2 ratio < 300, and/or lung infiltrates > 50% of the lung field within 24 - 48 hours) and in 6.1%, situations are critical (respiratory failure, septic shock, and/or multiple organ dysfunction/failure)] (6). Dyspnea and lung damage are caused by the host's immune response and cytokine release (cytokine storm) (7). Most clinical manifestations of COVID-19 include fever in approximately 80% of patients over the course of the disease (5), although it can be measured in 45% of the subjects (2). Research showed two-thirds of patients had coughs and one- third had sputum. Fatigue has been observed in 38.1% of patients (8). Dyspnea has been seen in 20% of cases. Less common symptoms are sore throat, myalgia, or arthralgia (14.8%) (6), chills, headache, nasal congestion, nausea, vomiting, diarrhea, and hemoptysis (2, 6). Mortality has been higher in patients with hematologic and other malignancies (9).
Patients with malignancy especially hematologic malignancies may be infected with more severe diseases. American society of hematology (ASH) published a study with 250 patients with hematologic malignancies. More common symptoms among patients were fever (73%), cough (67%), dyspnea (50%), and fatigue (40%). One-third of the patients had acute leukemia and 27 % had non-Hodgkin lymphoma. Overall mortality was 28 and 42% among moderate to severe infections. ASH concluded that these patients experience more severe forms of diseases and more mortality rates (10).
There is not enough data for managing acute myeloid leukemia patients with concomitant infection with SARS-COV-2. There are very few case reports and case series in this regard (11).
2. Case Presentation
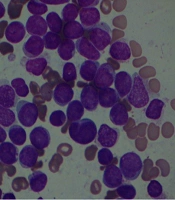
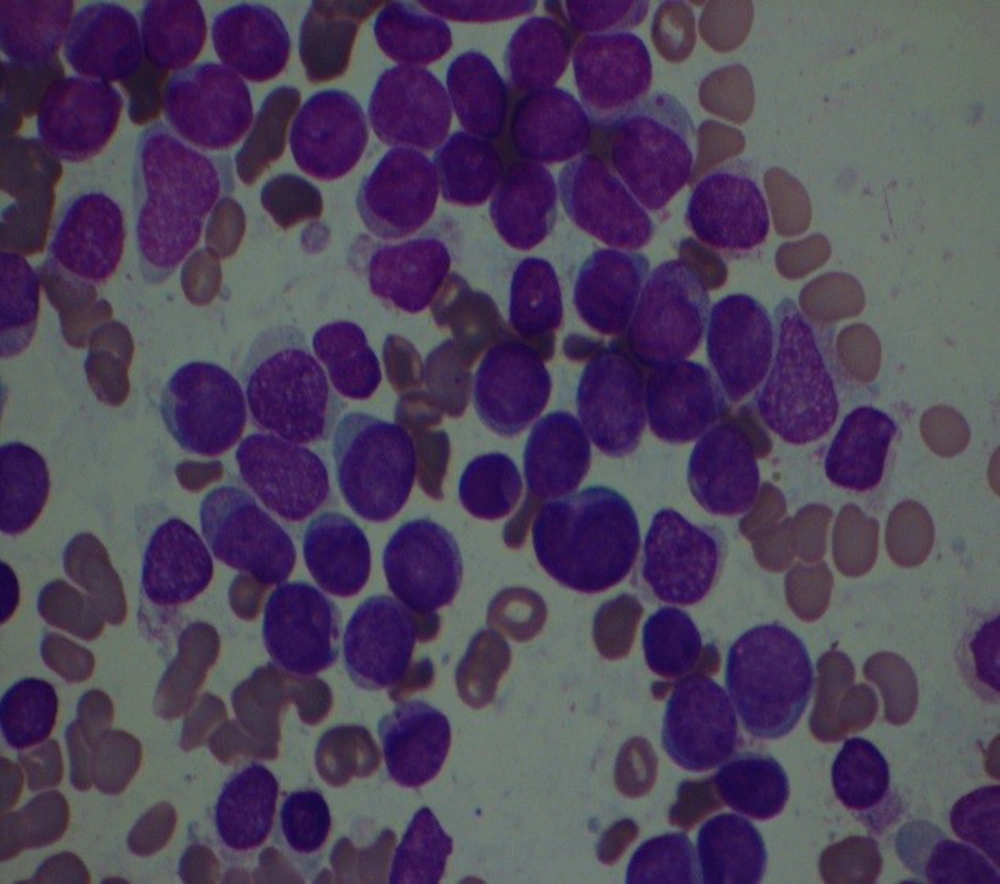
A 44-year man was suspicious of acute leukemia admitted to our hospital with complaints of cough and periumbilical pain on February 24, 2020. He was admitted to another hospital with a fever and excessive sweating on February, 9, 2020. Physical examination revealed right submandibular lymphadenopathy. In laboratory data he had pancytopenia. His chest X-ray was normal. Abdominal sonography showed multiple small stones in the gallbladder and his spleen's length was 137 millimeters. He has been receiving broad-spectrum antibiotics for about 10 days. Bone marrow biopsy revealed acute leukemia (Figure 1) and the patient was referred to our center for treatment.
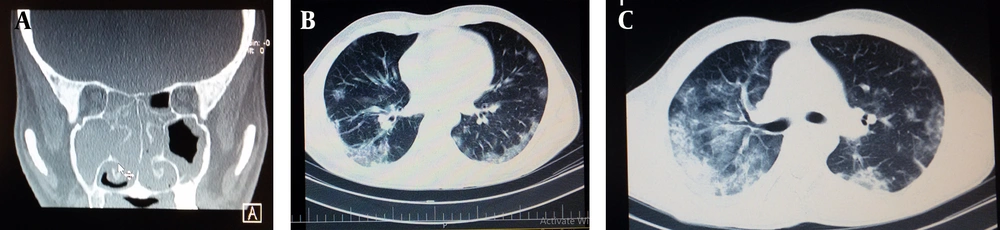
At the beginning of his admission, he complained of severe nasal discharge and dry cough. His past medical history was positive for chronic sinusitis. He was not febrile and other vital signs were normal. No significant lymphadenopathy and splenomegaly were found on physical examinations. Lung auscultation was normal. A paranasal sinus CT scan showed pansinusitis (Figure 2A). Clindamycin and ceftriaxone were started for him. On lab data, his white blood cells (WBC) were 2800 per cubic millimeter (mm3) and his platelets were 78000 per cubic mm. His urine analyses showed 15 - 20 red blood cells per high power field without pyuria. Table 1 shows some laboratory data of the patient. Flow cytometry from peripheral blood confirmed acute leukemia but could not reveal its type. On the fifth day of the admission when his WBC was 7800/mm3, a blood sample was sent to another laboratory in Tehran, Iran to repeat flow cytometry. It revealed a predominant myeloblast population of about 78%. Of the analyzed cells, 99% were positive for both CD34 and CD33. CD2 (21%) and CD64 (99%) were aberrantly expressed. Moreover, cMPO, CD19, CD20, and other markers were negative in analyzed blasts. As a result, acute myeloid leukemia with minimal differentiation (AML M0) was diagnosed.
A, CT scans of the patient; paranasal view shows pansinusitis; B, CT scans of the patient; chest CT scan on the 7th day of admission shows bilateral peripheral ground glass patchy infiltration; C, CT scans of the patient; chest CT scan before starting chemotherapy shows the progression of lung infiltrates when SPO2 was about 80 percent; D, CT scans of the patient; chest CT scan of the patient at the time of starting the second course of chemotherapy shows scanty residual pulmonary infiltrations.
| Lab Test | Value | Reference Value | ||
|---|---|---|---|---|
| 2020/2/24 | 2020/3/29 | 2020/30/2 | ||
| WBC/mm3 | 2800 | 36000 | 1200 | 4000 |
| Hemoglobin gr/dL | 11.5 | 7.7 | 7.3 | 13.5 - 17.5 |
| Platelet/mm3 | 78000 | 16000 | 66000 | 150 - 450000 |
| Alanine aminotransferase U/L | 48 | < 31 | ||
| Aspartate aminotransferase U/L | 54 | < 31 | ||
| Creatinine mg/dL | 1.1 | 1.8 | 1.2 | 0.3 - 1.3 |
| Lactate dehydrogenase U/L | 1014 | 480 | ||
| C-Reactive protein mg/L | 37 | < 6 | ||
| ESR (mm/h) | 85 | ≤ 20 | ||
Due to the intensity of dry cough and dyspnea, a chest X-ray was done on the sixth day that showed bilateral peripheral infiltrations at mid and lower lungs. subsequently, the chest CT Scan showed patchy bilateral peripheral ground glass infiltrations consistent with COVID-19 (Figure 2B). PCR for SARS-CoV-2 was not done. Antibiotics therapy was switched to meropenem and vancomycin. His axillary temperature increased to 38°C. Antiviral therapy with hydroxychloroquine, oseltamivir, and lopinavir/ritonavir (Kaletra) was started on the 7th day and lasted for 9 days. The patient's condition became worse and his oxygen saturation (SPO2) decreased to 80% while having a face mask. A chest high-resolution CT scan showed progressive disease (Figure 2C). On the 17th day of hospitalization, the WBC count increased to 35000, and hydroxyurea 4g divided daily dose was started. On the 18th day, WBC and platelet counts were 63800/mm3 and 16000/mm3. Chemotherapy with daunorubicin 60 milligrams per cubic meter of body surface area and cytarabine 200 mg daily continuous infusion was started and continued for 3 days. On the 22nd day, his breathing significantly improved (SPO2 increased to 94%) while his WBC count decreased to 1200/mm3. He could walk a few steps. Granulocyte colony-stimulating factor was started for him. Nadir for WBCs was 200/mm3 and for platelet was less than 10000/mm3.
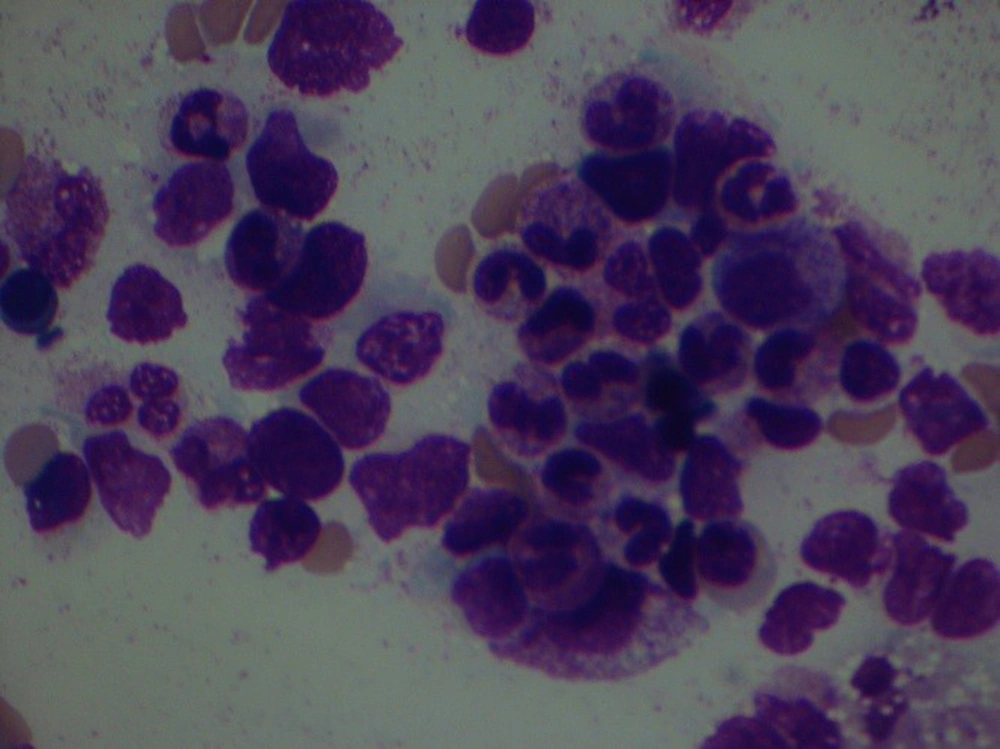
On the 12th day after chemotherapy (30th day) his WBC increased to 1200/mm3 and his creatinine was 0.9 mg/dL. Bone marrow aspiration was done which revealed complete remission of leukemia (Figure 3). The patient was discharged from the hospital on the 14th day of chemotherapy. Two weeks later he was admitted to the hospital for the second course of chemotherapy while his WBC was 3200/mm3, his neutrophils were 1840/mm3, his hemoglobin was 10.5 g/dL, and his platelets were 224000/mm3. His chest HRCT scan showed mild residual lung infiltrates (Figure 2D). The written informed consent was taken from the patient.
3. Discussion
Most hospitalized patients with SARS-COV-2 do have not any underlying disease (12, 13). Actually, underlying diseases are risk factors for severe COVID-19 disease (13). Overall, underlying diseases in hospitalized patients with COVID-19 vary in different studies from 31.7 to 51.5% (3). In a systematic review and meta-analysis, Emami et al. reported hypertension as the most prevalent [16% (95% CI: 10.15 - 23.65%)] underlying disease in hospitalized patients (14). The prevalence of other underlying diseases were estimated as: cardiovascular disease in 12.11% (95% CI: 4.40 - 22.75%), diabetes mellitus in 7.87% (95% CI: 6.57 - 9.28%), chronic kidney disease in 0.83% (95% CI: 0.37 - 1.43%), and malignancy in 0.92% (95% CI: 0.56 - 1.34%) of all the patients with COVID-19 (14).
Patients with cancer may be vulnerable to more severe forms of COVID-19. Zhang et al. reported 107 patients with cancer and COVID-19. Sixty-five percent of their patients, who were undergoing chemotherapy, were infected with severe forms of the disease (15). In a study, patients with recently diagnosed hematologic malignancies and COVID-19 within the past year had a 51.9% rate of hospitalization and a 14.8% mortality rate, significantly higher than patients with COVID-19 without hematologic malignancies (23.5, 5.1%, respectively) (P < 0.001) (16).
Our patient was firstly hospitalized because of an infection, probably due to immunosuppression of underlying acute leukemia. The infection with SARS-COV-2 may have occurred in the first hospital, because a Chest CT scan, that was taken approximately 3 weeks after the first admission, showed a typical picture of lung sequelae of COVID-19. Although dry coughs existed on the admission time, we considered postnasal discharge caused the symptom. Exaggeration of dyspnea was due to COVID-19, probably, because the chest CT scan showed progression of lung infiltrates. Leukostasis was less likely because WBCs gradually increased to 35000/mm3 on the 17th day, but the patient was suffering from severe dyspnea and was febrile and completely bedridden.
Hydroxyurea and chemotherapy decreased immune and myeloid blast cells. Rapid improvement in clinical condition and SPO2 were probably due to decreased cytokine levels. Dexamethasone was injected for 3 days with chemotherapy; it might play a role in the patient’s recovery; however, starting chemotherapy was life-saving.
Jin et al. reported a case of chronic lymphocytic leukemia and COVID-19 co-infection that was successfully treated with chlorambucil, intravenous immunoglobulin, and methylprednisolone (17). The outbreak of SARS-CoV-19 infection may cause delays in diagnosis and treatment of acute leukemia and may worsen the prognosis of the patients. A study on post-transplantation patients argued that, unlike common viral agents, coronaviruses do not cause more severe disease in immunosuppressed patients. However, according to another study, Centers for Disease Control and Prevention (CDC) did not agree with this idea (18). He et al. found that COVID-19 was not more prevalent in hematologic cancers; however, it causes more severe conditions and more deaths in these patients (19).
American society of hematology (ASH) recommends a routine 7 + 3 regimen for acute myeloid leukemia (20). However, our patient was treated with a modified regimen because of his critical condition and fortunately developed complete remission.
Based on more experience with hematologic malignancies and COVID-19, screening programs were prepared. Patients who need to go under transplantation or intensive and/or highly immunosuppressive therapy should be screened with PCR and chest CT scan. Patients with standard chemotherapy and less immunosuppressive therapy should be screened with PCR only (21).
3.1. Conclusion
Here we reported a unique case of acute myeloid leukemia infected with SASRS-CoV-2 that was successfully treated with chemotherapy and recovered from both diseases. We suggest that standard chemotherapy be initiated as soon as possible in cases of acute leukemia infected with SARS-CoV-2.