1. Background
Colorectal cancers are the third common malignancies after lung and breast neoplasms (1). However, there are multiple screening methods to reduce the morbidity and mortality of these cancers (2). Multiple risk factors including environmental and genetic issues have been involved in the pathogenesis of the disease (3). Dietary is one of the important environmental factors and studies have shown that a healthy dietary pattern can reduce the risk of colorectal cancer and colorectal adenoma, on the other hand, the “western” dietary pattern can increase the risks (4). The risk of postoperative recurrence rate is high) ranging from 4 to 27 percent (and preoperative chemotherapy is a useful method to improve the survival and reduce the recurrence (3). The main benefit of preoperative chemoradiation is complete clinical regression and pathological response (5). Pathological complete response (pCR), ranging from 10 to 30 percent, would increase the survival and decrease the recurrence rate (6). The pathological studies among patients with preoperative chemoradiotherapy have shown a significant reduction in the number and size of involved lymph nodes and frequency of lymph node metastasis (7). In this regard, there are different grading systems such as Mandard, Dowrak, Dowrak/Rodel, and tumor regression grading (TRG) (8). The outcome is related to multiple factors such as metastasis, size, lymph node involvement, and the like (9, 10). The standard treatment in locally advanced case is surgery, chemoradiotherapy, and chemotherapy (11). Safety, feasibility, and better resection are factors for preference of neoadjuvant chemoradiotherapy (12). It is usually used in cases with T3 and T4 tumors and sometimes in T1 and T2 tumors with lymphatic involvement (13). The pCR is defined according to both tumor and lymph nodes presenting sensitivity to treatment (14, 15). It is even useful in cases without response to discontinuing the treatment or increasing the intensity to achieve response and improvement in prognosis (16-20). Contributing factors for pCR include CEA level, anal verge distance, peripheral extension, smoking status, lymph node status, grade, size less than 5 cm, and time interval to surgery (21, 22). Despite various studies in this era, there are few studies on the location of tumors (21, 23-36).
2. Objectives
Regarding the high prevalence of colorectal cancer in Iran and the importance of neoadjuvant chemo-radiation for survival and morbidity, this study was carried out to determine the association between pathologic complete response and tumor location in patients with rectal cancer after neoadjuvant chemoradiotherapy.
3. Methods
3.1. Study Design
In this prospective descriptive comparative cohort, 100 cases with rectal adenocarcinoma from 2017 to 2019 were enrolled. Inclusion criteria consisted of stages 2 and 3, being candidates for neoadjuvant chemoradiation, +N/T3-4, no previous chemotherapy or hormone therapy or abdominal and pelvic radiotherapy, maximal age of 75 years, normal renal, hepatic, and hematological function. Exclusion criteria were dissatisfaction, loss to follow-up, simultaneous malignancy, and severe chemoradiotherapy adverse effects (If it causes the interruption in treatment).
All patients underwent radiotherapy at a dose of 45 grays in 28 fractions with capecitabine 825 mg/m2 twice daily and 5 times a week, and underwent surgery between 4 to 6 weeks after the completion of chemoradiation.
The pathologic complete response was defined as the absence of any cancer cells in the sample examined in pathology.
This study was approved by the ethics committee of Iran University of Medical Sciences: IR.IUMS.FMD.REC 1398.235.
3.2. Study Population
In this prospective study, the eligible cases were enrolled and the written informed consent form was received and demographic and clinical data were recorded in the checklist. Distance between anal verge and tumor was measured by clinical examination, colonoscopy, endo-sonography, and MRI. Tumors were defined as distal (less than 5 cm from the anal verge) and none distal (more than 5 cm from anal verge). Another subdivision was inferior (0 - 4.99 cm), middle (5 - 9.99 cm), and superior (10 - 15 cm). The pathological response was compared across the groups.
3.3. Statistical Analysis
Data analysis was done by SPSS software. The student’s t-test was used for numerical variables and the Pearson’s chi-square test was used for categorical factors. To determine the association between pCR and tumor location and calculation of odds ratio (OR), the logistic regression test was done. The P-values less than 0.05 were considered statistically significant.
4. Results
Among 192 studies cases, 100 patients including 24 female subjects were enrolled with a mean age of 55.2 (ranging from 23 to 70 years old). Also, the mean body mass index (BMI) was 23.87 (ranging from 19 to 27 kg/m2). The mean anal verge distance was 7.21 cm (ranging from 2 to 15 cm). Tumors were well, moderate, and poorly differentiated in 29%, 41%, and 30%, respectively.
Table 1 shows the association of 2-group location and variable, Table 2 shows the association of 3-group location and variables.
| Location, cm | P-Value | ||
|---|---|---|---|
| < 5 | ≥ 5 | ||
| Age | 0.304 | ||
| < 50 | 9/28 (32.1) | 19/28 (67.9) | |
| > 50 | 16/72 (22.2) | 56/72 (77.8) | |
| Family History | 0.517 | ||
| No | 22/86 (25.6) | 64/86 (74.4) | |
| Yes | 3/14 (21.40) | 11/14 (78.6) | |
| Grade | 0.417 | ||
| Well-differentiated | 9/29 (31) | 20/29 (69) | |
| Moderate differentiated | 11/41 (26.8) | 30/41 (73.2) | |
| Poorly and undifferentiated | 5/30 (16.7) | 25/30 (83.3) | |
| T stage | 0.134 | ||
| T2 | 7/18 (38.9) | 11/18 (61.1) | |
| T3 | 16/78 (20.5) | 62/78 (79.5) | |
| T4 | 2/4 (50) | 2/4 (50) | |
| N stage | 0.002 | ||
| N0 | 3/20 (15) | 17/20 (85) | |
| N1 | 18/50 (36) | 32/50 (64) | |
| N2 | 2/28 (7.1) | 26/28 (92.9) | |
| N3 | 2/2 (100) | 0/2 (0) | |
| Stage | 0.248 | ||
| II | 3/20 (15) | 17/20 (85) | |
| III | 22/80 (27.5) | 58/80 (72.5) | |
| Sex | 0.443 | ||
| Female | 7/24 (29.2) | 17/24 (70.8) | |
| Male | 18/76 (23.7) | 58/76 (76.3) | |
aValues are expressed as No. (%).
| Location | P-Value | |||
|---|---|---|---|---|
| < 5 | 5 - 10 | 10 | ||
| Age | 0.054 | |||
| < 50 | 12/28 (42.9) | 3/28 (10.7) | 13/28 (46.4) | |
| > 50 | 24/72 (33.7) | 25/72 (34.7) | 23/72 (31.9) | |
| Family History | 0.468 | |||
| No | 33/86 (38.4) | 23/86 (26.7) | 30/86 (34.9) | |
| Yes | 3/14 (21.4) | 5/14 (35.7) | 6/14 (42.9) | |
| Grade | 0.172 | |||
| Well- differentiated | 11/29 (37.9) | 8/29 (27.6) | 10/29 (34.5) | |
| Moderate differentiated | 17/41 (41.5) | 14/41 (34.1) | 10/41 (24.4) | |
| Poorly and undifferentiated | 8/30 (26.7) | 6/30 (20) | 16/30 (53.3) | |
| T | 0.031 | |||
| T2 | 12/18 (66.7) | 4/18 (22.2) | 2/18 (11.1) | |
| T3 | 22/78 (28.2) | 23/78 (29.5) | 33/78 (42.3) | |
| T4 | 2/4 (50) | 1/4 (25) | 1/4 (25) | |
| N | 0.001 | |||
| N0 | 5/20 (25) | 4/2 (20) | 11/20 (55) | |
| N1 | 26/50 (52) | 14/50 (28) | 10/50 (20) | |
| N2 | 3/28 (10.7) | 10/28 (53.7) | 15/28 (53.6) | |
| N3 | 2/2 (100) | 0/2 (0) | 0/2 (0) | |
| Stage | 0.141 | |||
| II | 5 (25) | 4 (20) | 11 (55) | |
| III | 31/70 (38.8) | 24/70 (30) | 25/70 (31.3) | |
| Sex | 0.251 | |||
| Female | 10/24 (41.7) | 9/24 (37.5) | 5/24 (20.8) | |
| Male | 26/76 (34.2) | 19/76 (25) | 31/76 (40.8) | |
aValues are expressed as No. (%).
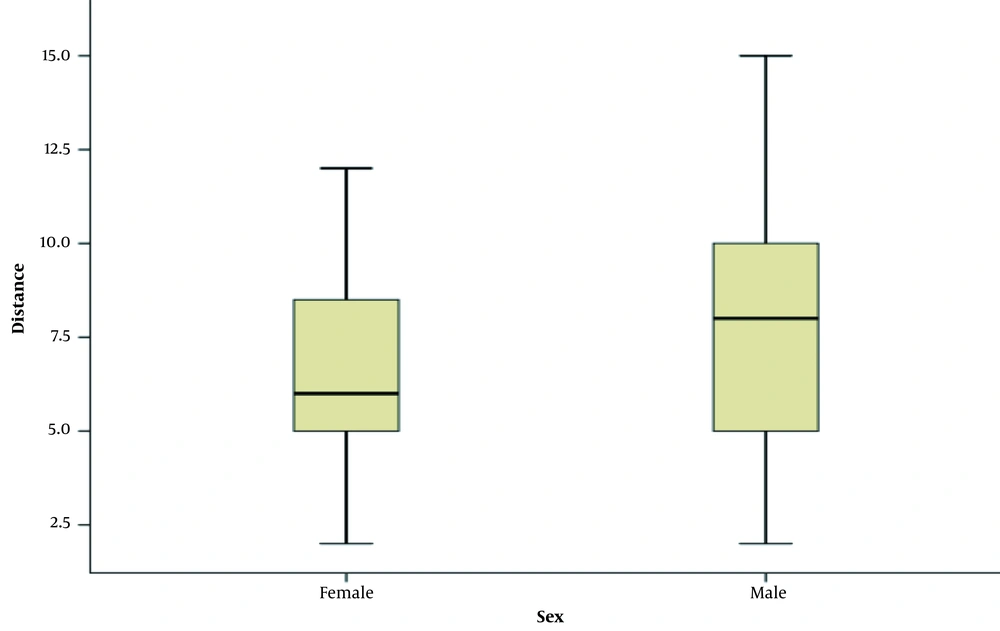
Also, 78% were T3 and 70% were N1/N2. Totally 20% were stage 2 and 80% were stage 3 (Table 3). The anal verge distance in two-third of cases was more than 5 cm. The distance by sex is shown in Figure 1.
| Response | P-Value | ||
|---|---|---|---|
| Response | No Response | ||
| Age group | 0.578 | ||
| < 50 | 13 (46) | 15 (54) | |
| > 50 | 29 (40) | 43 (60) | |
| BMI | 0.035 | ||
| < 25 | 36 (48) | 39(52) | |
| > 25 | 6 (24) | 19 (76) | |
| Family History | 0.080 | ||
| No | 39 (45) | 47 (55) | |
| Yes | 3 (21) | 11 (79) | |
| Grade | 0.014 | ||
| Well differentiated | 15 (51.7) | 14(48.3) | |
| Moderate differentiated | 21 (51.2) | 20 (48.8) | |
| Poorly and undifferentiated | 6 (20) | 24 (80) | |
| T | 0.369 | ||
| T2 | 10 (55.6) | 8 (44.4) | |
| T3 | 31 (39.7) | 47 (60.3) | |
| T4 | 1 (25) | 3 (75) | |
| N | 0.000 | ||
| N0 | 16 (80) | 4 (20) | |
| N1 | 20 (40) | 30 (60) | |
| N2 | 5 (18) | 23 (82) | |
| N3 | 1 (50) | 1 (50) | |
| Stage | 0.000 | ||
| II | 16 (80) | 4 (20) | |
| III | 26 (32.5) | 54 (67.5) | |
| Location | 0.031 | ||
| < 5 | 15 (60) | 10 (40) | |
| > 5 | 27 (36) | 48 (64) | |
| Location | 0.233 | ||
| 0 - 5 | 19 (52.8) | 17 (47.2) | |
| 5 - 10 | 11 (39.3) | 17 (60.7) | |
| > 10 | 12 (33.3) | 24 (66.7) | |
| Sex | 0.324 | ||
| Female | 8 (33.3) | 16 (66.7) | 0.324 |
| Male | 34 (44.7) | 42 (55.3) | |
Abbreviation: BMI, body mass index.
aValues are expressed as No. (%).
The anal verge distance was less than 5, between and 10, and more than 10 cm in 36%, 28%, and 36%, respectively. Despite 58% of without response cases, there were 30% and 12% with pCR and partial response, respectively.
The groups were well-distributed in terms of age, BMI, family history, grade, and T-stage. However, the rate of patients with advanced nodal involvement was higher in the non-distal group.
The groups were well-distributed in terms of family history, age, and grade. Although, the rate of patients with advanced nodal involvement and advanced T was higher in the non-distal group.
As shown in Table 4, age, grade, stage, N stage, and location were related to pCR.
| Response | P-Value | |||
|---|---|---|---|---|
| Complete Response | Partial Response | No Response | ||
| Age group | 0.038 | |||
| < 50 | 6 (21.4) | 7 (25) | 15 (53.6) | |
| > 50 | 24 (33.3) | 5 (6.9) | 43 (59.7) | |
| BMI | 0.065 | |||
| < 25 | 27 (36) | 9 (12) | 39 (52) | |
| > 25 | 3 (12) | 3 (12) | 19 (76) | |
| Family History | 0.065 | |||
| No | 28 (32) | 11 (12) | 47 (54) | |
| Yes | 2 (14) | 1 (7) | 11 (78) | |
| Grade | 0.006 | |||
| Well differentiated | 12 (41) | 3 (10) | 14 (48) | |
| Moderate differentiated | 17 (41) | 4 (9) | 20 (48) | |
| Poorly and undifferentiated | 1 (3) | 5 (16) | 24 (80) | |
| T | 0.574 | |||
| T2 | 8 (44) | 2 (11) | 8 (44) | |
| T3 | 21 (26) | 10 (12) | 47 (60) | |
| T4 | 1 (25) | 0 (0) | 3 (75) | |
| N | 0.000 | |||
| N0 | 9 (45) | 7 (35) | 4 (20) | |
| N1 | 17 (34) | 3 (6) | 30 (60) | |
| N2 | 3 (10) | 2 (7) | 23 (82) | |
| N3 | 1 (50) | 0 | 1 (50) | |
| Stage | 0.000 | |||
| II | 9 (45) | 7 (35) | 4 (20) | |
| III | 21(26) | 5 (6) | 54 (67) | |
| Location | 0.000 | |||
| < 5 | 15 (60) | 0 (0) | 10 (40) | |
| > 5 | 15 (20) | 12 (16) | 48 (64) | |
| Location | 0.001 | |||
| 0-5 | 18 (50) | 1 (2) | 17 (47) | |
| 5-10 | 9 (32) | 2 (7) | 17 (60) | |
| > 10 | 3 (8) | 9 (25) | 24 (66) | |
| Sex | 0.116 | |||
| Female | 8 (33) | 0 (0) | 16 (66) | 0.116 |
| Male | 22 (28) | 12 (15) | 42 (55) | |
aValues are expressed as No. (%).
As shown in Table 5, according to multivariate analysis, the anal verge distance was related to treatment response. According to Table 6, in multivariate analysis for 2-group treatment response the BMI, grade, and location were related to response.
| P-Value | Exp(B) | 95% CI for EXP(B) | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Response/ no response | ||||
| Grade | 0.035 | |||
| Grade (1) | 0.831 | 0.882 | 0.279 | 2.785 |
| Grade (2) | 0.026 | 5.040 | 1.217 | 20.867 |
| Location | 0.045 | 3.100 | 1.023 | 9.388 |
| N stage | 0.116 | 2.568 | 0.792 | 8.322 |
| BMI group | 0.288 | 2.047 | 0.546 | 7.669 |
| total stage | 0.001 | 10.574 | 2.471 | 45.243 |
| Constant | 0.000 | 0.000 | ||
| Complete Response | ||||
|---|---|---|---|---|
| P-Value | OR | 95% CI for EXP(B) | ||
| Lower | Upper | |||
| Step 1 | ||||
| Grade | 0.021 | |||
| Grade (1) | 0.861 | 0.901 | 0.281 | 2.888 |
| Grade (2) | 0.008 | 20.531 | 2.183 | 193.055 |
| Location | 0.003 | 6.224 | 1.896 | 20.435 |
| N stage | 0.026 | 4.529 | 1.198 | 17.128 |
| BMI group | 0.205 | 2.773 | 0.572 | 13.448 |
| Constant | 0.001 | .003 | ||
In multivariate and univariate analysis with considering the confounding effect of the N stage, in pCR, the anal verge distance was still meaningful.
5. Discussion
In this study, the pCR was seen in 30%. It ranged from 18% to 30% in previous studies (1). In univariate analysis BMI, grade, N-stage, and distance from anal verge were related to pCR. In cases with BMI over 25 kg/m2 and in tumors with low to intermediate grade, N0/N1, and distance less than 5 cm from the anal verge (low lying tumors) the pCR to neoadjuvant treatment was higher. Also, univariate analysis showed that BMI less than 25 kg/m2, low/intermediate grade, N0/N1, stage 2, a distance less than 5 cm from anal verge were related to tumor down-staging. In multivariate analysis tumor grade, N stage, and distance from anal verge were related to pCR. Also, tumor grade, total stage, distance from the anal verge, and N stage (P = 0.092) were related to tumor down-staging.
Various results were obtained in the other studies. In some studies pathologic complete response was more in distal tumor and in some other studies it was lower in distal tumors or there was no difference between pCR to neoadjuvant therapy and location of the tumor. Bitterman et al. (37) assessed 135 cases with T3-T4, locally unresectable T1-T2, low-lying T2, and/or node-positive rectal tumors with pCR in 26.3%. In multivariate analysis, CEA less than 5 cm, tumor size less than 3 cm, distance from anal verge less than 3 cm, the negative clinical node at diagnosis time, and time interval between surgery and chemoradiation more than 8 weeks were related to pCR. These results are in line with our findings.
In another study by Armstrong et al. (38) 885 cases with rectal tumors at stages 2 and 3 were assessed for contributing factors of pCR to neoadjuvant chemo-radiation. The pCR was seen in 18.2 percent. In multivariate analysis low CEA level, statin use, and less distance of rectal tumor from anal verge were significantly related to higher pCR (38). Das et al. (21) assessed 562 non-metastatic rectal cancer cases under chemo-radiation and surgical therapy and reported pCR in 19 percent and also 20 percent had a near-complete response. The circumferential extent was the only related factor for pCR and response was higher with less than 60 percent involvement. Circumferential extent less than 60 percent and distance from anal verge less than 5 cm were related to tumor down-staging (21).
The studies by Guillem et al. (39), Patel et al. (29), Restivo et al. (28), and Han et al. (32) were done among 109, 827, 260, and 332 cases, respectively and showed that distal rectal tumors had lower pCR (39). Also, the pCR was not related to anal verge distance in some other studies. Some possible causes for the difference in results include retrospective design, selection bias, small sample size, and lack of adjustment for confounding factors such as grade, radiotherapy dose, chemotherapy protocol, stage, aspirin/statin use, radiotherapy length, and time interval between surgery and chemo-radiation termination time, and also genetic/racial differences. Despite the novel improvements in the management of cancers such as the discovery of new molecular targets and subsequently new drugs (40-43), considering the basic topics such as the location of the tumor might provide not only new data for the management of these cancers especially in countries with limited resources but also provide the new concepts for further studies.
5.1. Conclusions
The findings of the current study showed that there may be some association between rectal tumor location and pathologic complete response. The main limitations of our study were retrospective design, loss to follow-up cases, lack of clinical staging by a single radiologist, and lack of re-assessment of slides by a single pathologist (inter-observer bias), no access to data about the distance between chemo-radiation and surgery, and also small sample size. Further studies with a larger sample population and alleviation of these limitations are encouraged to attain more definite results.