1. Introduction
Ewing sarcoma (ES) is a highly aggressive round cell tumor that lacks cellular or structural differentiation. ES is the second most common malignant bone tumor in children and young adults that usually shows a high rate of local recurrence and distant metastasis (1).
Extraosseous Ewing sarcomas (EESs) are rare tumors that originate from soft tissues and upper extremity EESs account for about 3% of all cases (2).
We reported a 27-year-old female with EES of the right deltoid region that unfortunately, management of her potentially curable disease became complicated due to the COVID- 19 pandemic.
2. Case Presentation
A 27-year-old female presented with progressive swelling and pain in the right deltoid for more than 4 months. As her signs and symptoms had begun following minor trauma, and besides, due to the COVID-19 outbreak, she had more than 3 months delay in seeking medical advice.
Her physical examination revealed a 20 × 15 cm firm and tender swelling over the left deltoid region that has caused moderate pain and severe limitation on range of the motion.
Other parts of the physical examination were unremarkable and all her laboratory tests were within normal limits.
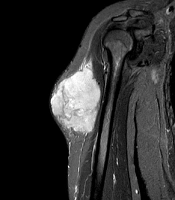
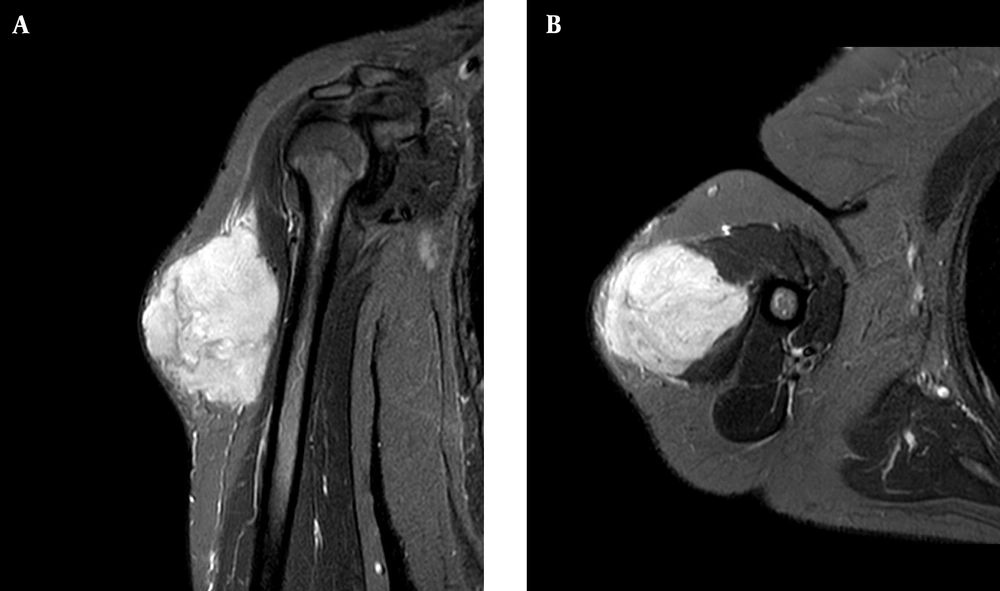
Magnetic resonance imaging (MRI) of the affected arm showed a well-defined hyperintense hemorrhagic mass lesion in the lateral part of the upper muscle of the arm, highly suspicious of a malignant legion with the normal fat plane and adjacent bone (Figure 1A and B).
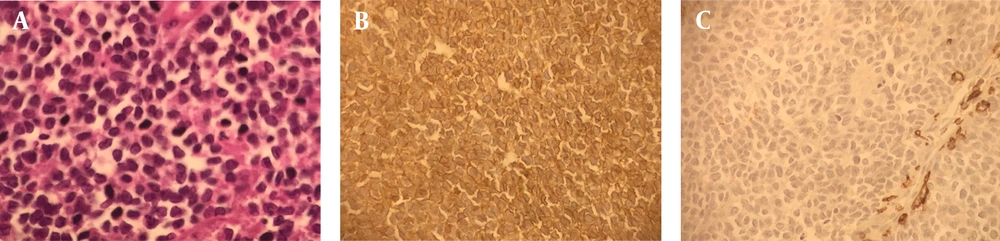
A biopsy was taken, and the final diagnosis of Ewing sarcoma was made (Figure 2A - C). Metastasis work-up showed no metastatic lesion. Neoadjuvant chemotherapy with VAC-IE regimen (vincristine, doxorubicin, and cyclophosphamide, alternating with ifosfamide, and etoposide every 3 weeks) was started. The lesion responded well to the treatment with more than 50 % size reduction after 2 cycles of chemotherapy.
A, sheets of small round cells with scant cytoplasm and indistinct cell membranes having minimal amount of stroma (microscopic image at x40); B, tumor cells showing membrane positively for CD 99 (microscopic image at x40); C, tumor cells are negative for lCA ,cytokeratin, chromgranin and synaptophysin
During the third cycle, the lesion began to pain and grow. We discussed this case in the multidisciplinary meeting and finally, we decided that she should undergo surgery.
Since all the elective surgeries at that time were canceled due to the COVID-19 pandemic, chemotherapy was resumed with a different regimen (irinotecan and temozolomide), while she was still non-metastatic based on re-staging scans.
Following the second course of the second-line chemotherapy, she developed neutropenic fever and was hospitalized for 10 days.
Two weeks after the discharge, in the scheduled surgery time, she was infected with COVID-19 and was admitted to the intensive care unit (ICU) due to the severe symptoms. She was discharged after 22 days and stayed at home for more than 20 days because of poor performance status. Meanwhile, the lesion began to grow again and it measured 40 × 30 cm when she was admitted for surgery.
Her surgical treatment was canceled once more due to a lack of ICU beds.
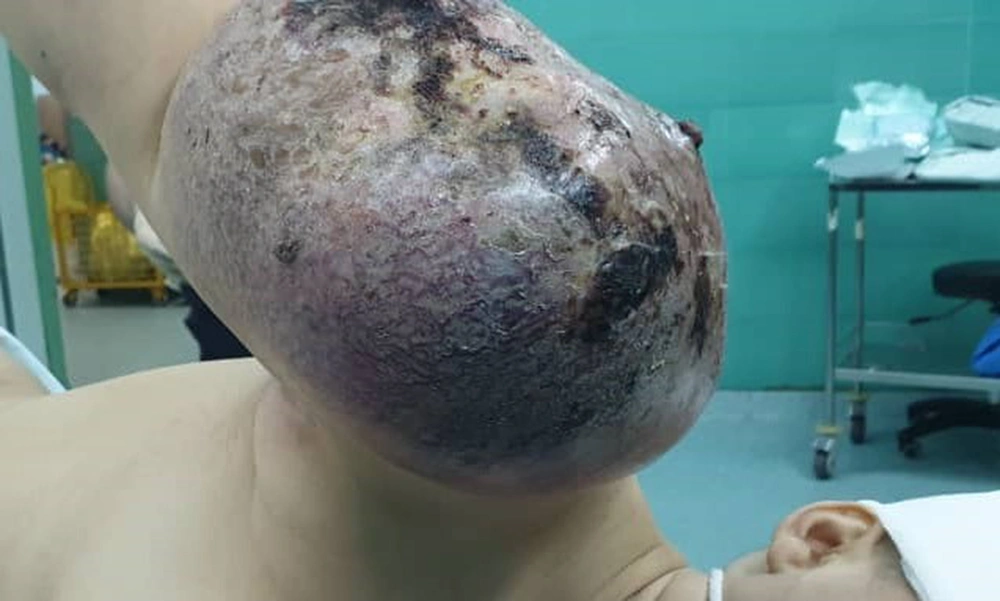
Finally, about 5 months after the initial visit, right shoulder disarticulation surgery was performed for her (Figure 3).
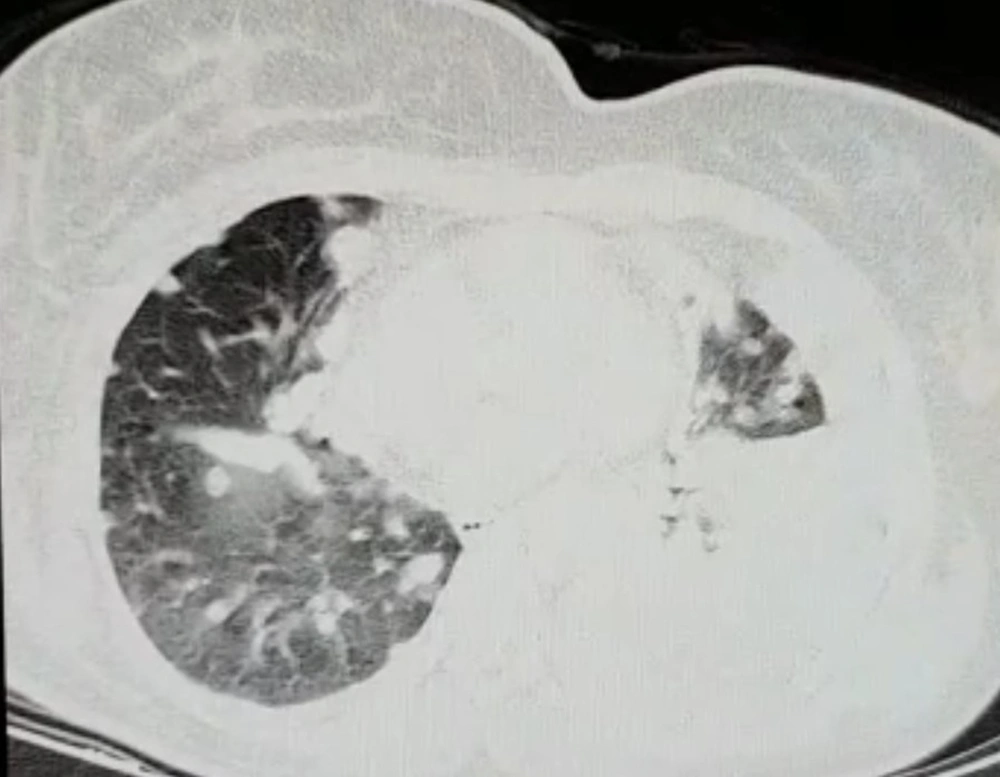
After the surgery, she stayed in the ICU for 10 days due to respiratory distress. About 3 weeks following the surgery she was well enough to continue the chemotherapy. The chemotherapy was resumed with the same regimen as the second-line treatment. During the second course, she developed respiratory distress and the chest computed tomography (CT) scan revealed multiple metastatic lesions in both lungs as well as the residual ground-glass opacities of the COVID-19 infection (Figure 4). She was admitted to the ICU and passed away 12 days afterward.
3. Discussion
In this paper, we reported a rare but potentially curable case of EES whose management was complicated due to the COVID-19 pandemic.
Ewing sarcoma is a poorly differentiated, highly malignant and round cell tumor which lacks cellular or structural differentiation (3). Although, it is primarily a bone tumor, a large-scale analysis of the surveillance, epidemiology and end results (SEER) database demonstrated that up to 31% of cases might arise from extra-skeletal tissues (4).
Extraosseous Ewing sarcomas are rare tumors that originate from soft tissues and most often involve paravertebral spaces, lower extremities, head and neck, and pelvis and upper extremity EESs account for about 3% of all cases (2-4).
Signs and symptoms of EES depend on the site of origin and is commonly due to the mass effects, including pain and swelling (4).
The presentation of EES is somewhat different from that of osseous ES. While ES generally presents in the second decade of life with a slight female predilection, EES has a bimodal age distribution and is commonly found in patients younger than 5 years and older than 35 years, with no gender preference (4, 5).
Radiographic findings of EES include increased soft tissue density without any bony involvement with calcification in about 25% of the cases. MRI is useful in evaluating the extent of the tumor and its relation with the neurovascular bundles. On MRI, EES is often of low to intermediate signal intensity on T1-weighted images and of high signal intensity on T2-weighted images and shows heterogeneous contrast enhancement (1-3).
One important characteristic of EESs is that although they can cause cortical erosion and/or a periosteal reaction, actual osseous involvement is rare, even when the mass is located near the bone (3).
The recommended treatment for any member of the Ewing tumor family is local treatment with surgery (or radiotherapy for inoperable cases) plus systemic therapy. Based on the most international clinical guidelines, neoadjuvant chemotherapy followed by aggressive surgical excision with or without radiation therapy is the preferred treatment method for the majority of the patients (1, 3).
By standard treatment, ES and EES have similar prognosis in general, with 5-year overall survival (OS) of 65 to 75% for localized disease, and less than 30% for metastatic disease (6-8). However, some studies have reported better event-free and overall survival rates for EES (9).
Timely commencement of systemic therapy along with aggressive local therapy (mainly surgery, and in some cases radiotherapy) are of paramount importance in achieving survival benefits. One study on 27 patients with EES reported that patients who had more than 90% tumor necrosis (less than 10% viable tumor) had a 100% 5-year OS. Besides, patients who received induction chemotherapy and underwent wide surgical resection with negative margins had also a 100 % 5-year OS (10).
According to the majority of international clinical guidelines, we started our patient’s treatment with neoadjuvant chemotherapy with the hope of controlling the systemic disease, as well as increasing the chance of limb preservation and achieving negative surgical margins. Unfortunately, the COVID-19 pandemic took her medical management out of our control to a great extent.
Our patient initially had a 3-month delay in seeking medical advice due to COVID-19 fear. Based on the recently published data, every 4 weeks delay in diagnosis and treatment of patients with different types of cancers, can result in a 6 to 13% higher risk of death (11-13).
Furthermore, reports demonstrated that patients with cancer not only are at greater risk of contracting COVID-19 but also are more susceptible to develop severe infection and as a result, have a significantly higher mortality rate from COVID-19 (14).
This high risk of mortality could be due to immunosuppression, increased co-existing medical conditions, and pulmonary compromise in cases of involvement of lungs with malignancy (14).
In addition, experts suggest that severe COVID-19 may create a microenvironment favorable to cancer recurrence by immune-mediated tumor reawakening (15).
COVID-19 can activate several factors that have been previously shown to have a role in tumorigenesis and metastatic relapse. Recent studies have revealed that COVID-19 can target common cancer pathways including those involved in cell cycle progression, metabolism, and epigenetics (15). This finding will further complicate the interaction between COVID-19 and malignant diseases.
It can be assumed that several factors including delayed diagnosis and treatment commencement, higher susceptibility of contracting COVID-19, treatment interruptions due to shortage of hospital bed and healthcare personnel, more severe respiratory infection due to malignancy, the possible role of COVID-19 in tumor reawakening, and finally, aggressive behavior of her cancer, altogether prevented us from achieving our desired treatment result and led to the patient death.
3.1. Conclusions
Extraosseous Ewing sarcoma is a rare but curable disease. COVID-19 pandemic has affected all aspects of medical care including the management of patients with cancer. Although not “emergent” by definition, surgical treatment of patients with cancer, especially those who suffer from malignancies with high metastatic potential such as Ewing sarcoma (including EES) should not be considered as “elective”, as the disease may progress in a short time and become incurable.