1. Background
Breast and thyroid cancers remain among the most common malignancies in women, accounting for 24.2% and 5.1% of all cancers, respectively. The increasing trends of breast and thyroid cancers around the world, especially among young women, necessitate evaluating some screening programs to reduce the burden of these common diseases (1). The incidence of thyroid cancer in Iran, which is estimated at 2.17 per 100,000 people, is also following an upward trend (2).
An incidental thyroid nodule (ITN) is common ultrasonography (US) finding in healthy middle-aged women. In previous studies, the prevalence of ITN was estimated at 41.7% in asymptomatic women, referred for their regular checkups. The higher level of healthcare access for the general population may be responsible for the elevated prevalence of thyroid nodules (3). Some studies reported that ITNs are benign and clinically unimportant, while others suggested US screening for the early detection of thyroid cancer.
So far, the US rationale for routine population screening has been controversial (4, 5). Advances in breast cancer diagnosis and treatment can lead to a higher rate of secondary malignancies in these patients. The incidence of thyroid cancer, as a secondary malignancy to breast cancer, is higher than expected (odds ratio [OR]: 1.44 - 1.67). This association may be attributed to the role of hormones, radiation exposure, genetic susceptibility, psychological distress, and shared risk factors between thyroid and breast cancers (6). Psychological stress from cancer diagnosis may also increase the risk of other malignancies and must be considered in the follow-up of patients (7, 8). The US examination can be used as a sensitive modality for detecting malignant nodules (9).
To define the malignancy risk of a nodule, the thyroid imaging, reporting, and data system (TI-RADS) have been established as a standard reporting system, based on different factors including echogenicity, echogenic foci, shape, margin, and composition of the thyroid nodule (10). The US risk stratification of thyroid nodules shows high sensitivity and a high negative predictive value for detecting thyroid carcinomas; this method can be complemented with history-taking and fine needle aspiration (FNA) (11, 12).
2. Objectives
This study aimed at evaluating the risk and incidence of ITNs in patients with breast cancer versus healthy controls using the TI-RADS and investigating the use of US screening for patients with breast cancer.
3. Methods
3.1. Ethical Considerations
This study adhered to the Declaration of Helsinki ethical guidelines, as well as national and international guidelines. The Institutional Review Board of Shahid Beheshti University of Medical Science approved the study, and written informed consent was obtained from the subjects.
3.2. Study Design
In this single-center, cross-sectional study, a total of 140 patients with breast cancer, confirmed by the pathology results of core needle biopsy, were included. Moreover, 140 women, in a similar age range, who were visiting the radiology department of Imam Hossein Hospital for their breast checkup, were included in the control group. The control group was cancer-free, based on mammography or breast US findings.
The exclusion criteria for the case and control groups were as follows: a history of radiation therapy, undergoing chemotherapy, and unwillingness to participate in the study. A radiologist with 7 years of experience performed thyroid sonography. The thyroid investigation of patients with breast cancer was carried out before the onset of treatment at the time of diagnosis. The researchers gathered data in a single-blind fashion.
3.3. Data Collection
A medical student completed the questionnaires in an in-person interview before the thyroid US examination. The questionnaire included age, body mass index (BMI), marital status, smoking, menopausal status, and thyroid disease history. The type of breast cancer was determined based on the pathology report.
3.4. US Examination
An experienced radiologist examined the thyroid gland, using a hand-held 6.6 - 11 MHz linear transducer. The US findings indicated the volume of each lobe (volume = length × width × height × 0.479), as well as thyroid abnormalities on the US image (e.g., uniformity, nodular goiter, multinodular goiter, and Hashimoto’s disease). Thyroid nodules were defined as “discrete lesions within the thyroid gland and radiologically distinct from the surrounding thyroid parenchyma” (13). A lesion, with the solid portion comprising 6 to 100% of its total volume, was defined as a nodule. The length, depth, and width of each nodule were measured at their longest aspect on the US image. Moreover, the risk stratification of thyroid nodules was performed, based on the American College of Radiology (ACR) TI-RADS. As shown in Appendix 1, the TI-RADS is a scoring system that quantifies the thyroid sonographic characteristics to estimate the malignancy risk of nodules (10).
3.5. Statistical Analysis
Continuous variables are presented as mean ± standard deviation (SD), and categorical data are shown as numbers or percentages. Kolmogorov-Smirnov test confirmed the normal distribution of the participants’ age. Besides, a Chi-square test was performed to compare qualitative variables. The student’s t-test and Fisher’s exact test were also used for comparing quantitative variables and mean values. The significance level (α) was considered to be 0.05 in all tests. Data analysis was performed in SPSS version 24.0 (released in 2016, IBM SPSS Statistics for Windows, IBM Corp. Armonk, NY, USA).
4. Results
The mean age of the participants (n = 280) was 42.73 ± 6.16 years, and there was no significant difference between the case and control groups (P = 0.094). Also, the BMI of women in the case and control groups was not significantly different (P = 0.052). Besides, there was no significant difference in the right or left lobe volume between cancer and healthy groups (P = 0.211 and P = 0.054, respectively) (Table 1).
| Variables | Breast Cancer Patients (n = 140) | Healthy Control (n = 140) | P-Value |
|---|---|---|---|
| Age (y) | 43.35 ± 7.85 | 42.11 ± 3.69 | 0.094 |
| Body mass index (BMI) (kg/m2) | 26.33 ± 3.64 | 25.63 ± 2.16 | 0.052 |
| Right lobe volume (mL) | 3.87 ± 1.86 | 3.61 ± 1.58 | 0.211 |
| Left lobe volume (mL) | 3.35 ± 1.67 | 2.97 ± 1.59 | 0.054 |
| Menopause | 26/140 (18.6) | 21/140 (15) | 0.424 |
| Smoking | 18/140 (12.9) | 11/140 (7.9) | 0.170 |
| History of thyroid disease | 13/140 (9.3) | 6/134 (4.3) | 0.154 |
| Type of cancer | |||
| Ductal carcinoma in situ | 20 (14.3) | ||
| Invasive ductal carcinoma | 114 (81.4) | ||
| Lobular carcinoma | 6 (4.3) | ||
| Thyroid US examination | 0.001 | ||
| Normal | 50 (35.7) | 107 (76.4) | |
| Uniformity | 6 (4.3) | 3 (2.1) | |
| Nodular | 72 (51.4) | 26 (18.6) | |
| Multinodular | 8 (5.7) | 2 (1.4) | |
| Hashimoto’s disease | 4 (2.9) | 2 (1.4) |
Comparison of the Participants’ Characteristics Between the Case and Control Groups a
The analysis of the participants' characteristics showed that 18.6% and 15% of them were menopausal in the case and control groups, respectively. There was no significant difference between the 2 groups regarding menopause or smoking status (P = 0.424 and P = 0.170, respectively). The analysis of thyroid disease history data showed that 9.3% of the patients in the case group and 4.3% of the participants in the control group had a thyroid disease history; however, the difference among the groups was not significant (P = 0.154). Overall, the characteristics of the 2 groups were not significantly different (Table 1). Invasive ductal carcinoma was the most frequent type of breast cancer (81.4%) in the case group, based on pathology reports (Table 1).
The US findings in the case and control groups showed that 76.4% of the subjects in the control group underwent a regular thyroid examination; however, only 32.9% of the patients in the case group had a normal thyroid status (P < 0.0001). Considering the frequency of nodules, 51.4% of the patients in the case group and 18.6% of the participants in the control group had thyroid nodules in the US images; there was a significant difference between the case and control groups (Table 1).
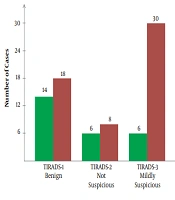
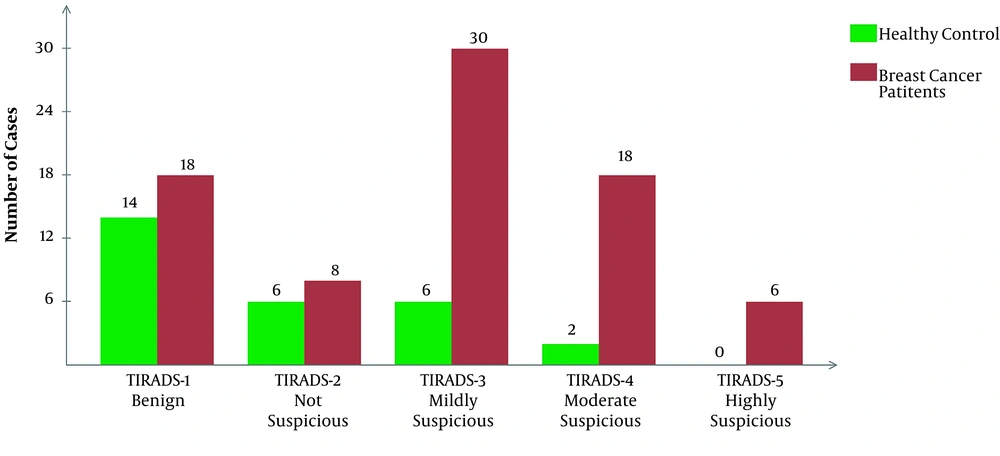
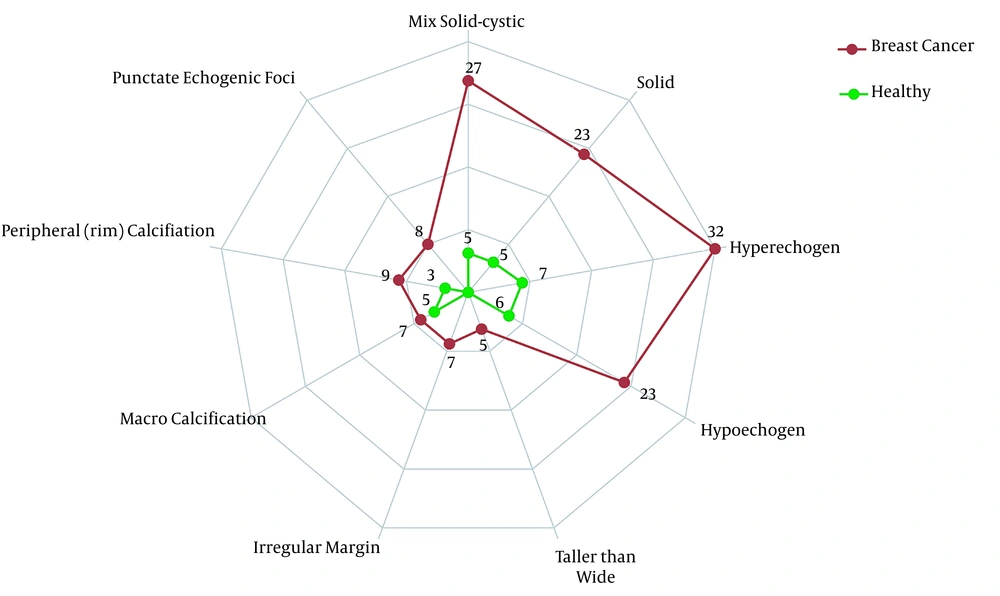
The frequency of all five TI-RADS scores was significantly higher in the case group compared to the control group (P < 0.0001). There were no healthy participants with TI-RADS-5 nodules, whereas the frequency of these nodules in patients with breast cancer was 5.7%, which indicates a significant difference between the 2 groups. Similar results were reported regarding the frequency of multinodular goiters between the case and control groups. The frequency of nodules and TI-RADS scores are presented in Figure 1. The features of thyroid nodules are also shown in Figure 2. Hyperechogenic, mixed cystic-solid components were among the most common features of patients with breast cancer; also, solidity and hypoechogenicity were the other dominant features.
5. Discussion
The aim of the present study was to evaluate the incidence rate and malignancy risk of ITNs in patients with breast cancer, using TI-RADS. Recent studies have reported a high incidence of thyroid nodules in the general population, which may be due to increased access to healthcare services and limited cases of nodule progression into malignancy. Therefore, the use of US screening of thyroid nodules is indeterminate for identifying nodules at a higher risk of malignancy in the general population (3, 4). The current study showed a higher incidence of thyroid nodules, along with a higher malignancy risk, in patients with breast cancer compared to the healthy participants. Therefore, routine US screening may be beneficial in the follow-up of diagnosed patients with breast cancer.
In a previous study, women with a history of breast cancer were almost 3 times more likely to develop thyroid cancer (14). In agreement with previous research, we also found more US abnormalities in patients with breast cancer. TI-RADS-4 and TI-RADS-5 were detected in 15% of the patients, demonstrating a moderate to the high probability of malignancy. Moreover, a study, performing US examinations of the thyroid gland for screening thyroid gland abnormalities and FNA for 10% of the patients, reported a higher incidence of pathologically proven thyroid malignancies in patients with breast cancer (1.9% vs. 0.6%) (15).
Besides, Park et al. reported a higher incidence of thyroid cancer among patients with breast cancer (2.5%). In their study, there was a significant difference in the frequency of thyroid cancer at the time of breast cancer diagnosis between patients with breast cancer and the healthy controls (16). According to previous reports, the incidence of radiation-induced thyroid cancer usually increases as the time interval from the onset of therapy increases (17). Moreover, a meta-analysis confirmed the relationship between these two common diseases (6). Therefore, patients with breast cancer are at a higher risk of developing thyroid cancer as a secondary disease, and hormonal and genetic factors, besides treatment, may play a role in this relationship. However, the cause of this relationship is not clearly known (18-20).
The coincidence of thyroid disease with breast cancer has been a controversial subject for a long time. The mammary glands have fatty tissue, containing thyroid-stimulating hormone (TSH) receptors; therefore, the possible interactions between the thyroid and breast tissues may be related to these receptors (21). Moreover, endocrine stimulation by thyroid products may have simultaneous effects on the breasts (22, 23). A study by Turken et al. reported the increased prevalence of autoimmune and non-autoimmune thyroid diseases in patients with breast cancer and found an increase in thyroid peroxidase antibodies (24). A novel explanation for the coincidence of thyroid and breast cancers is the reduced energy of cells, altering the cancerous tissue (25).
So far, the US examinations have been used as a predictor of thyroid cancer; however, some overlaps in the appearance of US abnormalities have led to the prioritization of FNA. Nonetheless, the US remains a crucial, available, and non-invasive modality for investigating the thyroid gland (26). TI-RADS, a well-known scoring system, which assesses the malignant risk of thyroid nodules, may help physicians rate thyroid nodules more accurately and prevent unnecessary biopsies. Our US examination of the thyroid gland showed a significantly higher rate of abnormalities in newly diagnosed patients with breast cancer. The TI-RADS scores were drastically higher in patients with breast cancer, as shown in Figure 1. Also, the characteristics of nodules are presented in Figure 2. In brief, ITNs were more prevalent and malignant among patients with breast cancer. Therefore, the present results are in line with previous studies, which showed thyroid changes in patients with breast cancer.
In the present study, there was no significant difference in terms of age between the case and control groups. We also investigated thyroid nodules before the onset of treatment (i.e., chemotherapy or radiotherapy). Therefore, age and radiation cannot explain this difference, while genetic factors, hormonal factors, or similar risk factors between these 2 cancers may explain this difference. Considering the increasing trend of thyroid cancer in Iran, early detection of thyroid malignancies and screening programs can be beneficial (27).
The data on BMI, age, and menopausal status showed no significant difference between the case and control groups. According to previous studies, the relationship between thyroid disease and BMI is not entirely clear, although BMI might affect the thyroid gland (28). Besides, previous studies have shown that the incidence of thyroid disorders increases along with advancing age (29). However, there is not enough evidence regarding the relationship between menopause and thyroid diseases. On the other hand, by increasing our understanding of this relationship, we can reduce the outbreak of some thyroid diseases, especially autoimmune diseases (30, 31). Additionally, earlier studies have suggested the effect of estrogen on the thyroid gland; therefore, the association of breast cancer with menopausal status and thyroid disease may be attributed to estrogen (32). Similarities in BMI, age, and menopausal status of the case and control groups prevented the possible confounding effects.
One of the main limitations of this study was the lack of FNA of thyroid nodules owing to the patient’s unwillingness to undergo FNA, probably due to the detection of a new cancer, which raises concerns about the disease and its treatment course. The potential bias of cross-sectional studies (i.e., recall bias and selection bias), the limited number of participants, the operator-dependent nature of US examinations, and the absence of a second radiologist for these examinations are the other limitations of this study. Future studies are suggested using the gold standard diagnostic tools compared to TI-RADS to examine the specificity and sensitivity of TI-RADS for early detection of thyroid cancer in early breast cancer patients.
5.1. Conclusions
In the present study, patients with breast cancer had more thyroid abnormalities in the US examinations. The drastically higher TI-RADS score in patients with breast cancer represents a higher risk of malignancy compared to healthy individuals. Overall, patients with breast cancer may benefit from a regular sonographic investigation of the thyroid gland.