1. Background
Among the conventional therapeutic procedures of cancer, sonodynamic and photodynamic therapy (SPDT), which incorporates a combination of ultrasound, laser, and a sensitizer agent offered a route toward a non-invasive method. Photodynamic therapy (PDT) is an inventive minimally invasive therapy that is applied as a substitute protocol for the management of cancer. It is based on the buildup of a nontoxic photosensitizer (PS) that is excited by light and reacts with oxygen to generate singlet oxygen, radicals, and triplet species in tumor tissue, the consequence of which is the initiation of cell death mechanisms (1, 2). The photosensitizer is one of the basic components of PDT, despite light and oxygen (2). Accidentally discovered PDT by Dougherty has proved the value of porphyrin compounds in oncologic suitable photosensitizers (3). The first generation of PDT sensitizers that was approved by the Food and Drug Administration (FDA) (Photofrin) produced singlet oxygen for cancer treatment (4). PDT has not caused DNA damage, mutations, and carcinogenesis, since most PSs do not accumulate in cell nuclei. Although PDT is very safe in the tissues around the cancer region, the light penetration depth is limited and often produces less success (5). Therefore, the wavelength range between 600 and 800 nm with depth penetration of about 8 mm into the tissues has been determined as the practical “therapeutic window” for clinical PDT. Lasers as a standard light source for PDT are monochrome and have high luminance (6). The findings of Banerjee et al.'s study confirmed a potential role for PDT with verteporfin and laser (690 nm) in the management of early breast cancer in 12 female patients (1). Secret et al. proposed that PDT with porphyrin delivery by porous silicon nanoparticles can improve the treatment of breast cancer cells (7, 8).
Since 1989, a novel promising non-invasive approach based on PDT was established with similar principles to PDT. Sonodynamic therapy (SDT) involves the use of ultrasound radiation and a sonosensitizer agent that can be activated by ultrasound. Therefore, SDT overcomes the major limitation of PDT (9). On the other hand, SDT as a non-invasive method for cancer treatment has a deeper penetration ability into the cancer tissue and effectively increases cytotoxicity (10). In SDT, ultrasound exposure utilizes an appropriate frequency and intensity (1 - 3 MHz, 0.5 - 3 W/cm2). These waves interact with sonosensitizing agents and as a result, produce free radicals, which cause apoptosis of cancer cells. This activation is related to the cavitation process (11-13), which involves the formation, growth, and exploding of gas-filled bubbles in fluids (9, 14).
As an SDT sensitizer, hematoporphyrin (HP) can maximize the ultrasound effects. On the other hand, porphyrin-based molecules are easily condensed into physiological environments due to their low water solubility (15). Nanoparticles, as a drug delivery system, can easily penetrate cell dams (such as membranes) and effectively accumulate the drug within the tumor tissue. Mesoporous silica nanoparticles (MSNs) have been considered within the field of treatment and diagnosis. For the discharge of the drug-loaded into the mesoporous nano-carriers, the external stimulus of ultrasound is extremely much considered because, in addition to activating sensitivities, it allows the spatial and temporal control of drug release at the specified location, hence increasing therapeutic benefits (16-18). Nevertheless, in a hypoxic tumor medium, the competency of SDT and PDT is low that limits their applications (19). To overcome the aforementioned limitations of these two treatment modalities, the combination of SPDT can help to get a reasonable anti-tumor effect because of the ultrasound good tissue penetration, and focusing of its energy into the specific depth of biological tissue (4).
The sono-photodynamic therapy (SPDT) depended on the accumulation of sensitizer in tumor sight and cytotoxicity enhancement after activation by light or ultrasound. Besides the singlet oxygen production in PDT, mechanical stress, cavitation, and multiple reactive oxygen species are generated in SDT (4). It is concluded that dual-frequency ultrasound produces more active bubbles and enhances cytotoxic effects when compared with a single-frequency ultrasound. Dual-frequency ultrasound causes the drug to discharge from the carriers and increases drug release into the aqueous environment, which increases cancer cell death (20-22).
2. Objectives
SPDT performance, as a minimally invasive cancer treatment, was dependent on the accumulation of sensitizer in tumor sight and cytotoxicity enhancement after activation by ultrasound and laser radiation. The aim of the current research was to evaluate the effects of Hematoporphyrin-mediated SPDT in the management of breast adenocarcinoma. Therefore, the therapeutic effect of sensitized HP and HP-MSNs with the combination of dual-frequency ultrasound (1 and 3 MHz) and laser light (650 nm) in the treatment of Inbred Balb/C mice grafted breast adenocarcinoma were assessed. Tumor volume was measured during 30 days, and the parameters related to tumor growth, animal survival, and histopathological study were recorded.
3. Methods
3.1. Chemicals
HP 50% (Sigma-Aldrich, Canada) was dissolved in phosphate-buffered saline (PBS, pH = 7.4) and stored in the dark at 4°C. The synthesis of MSNs was performed in the sol-gel process by application of an alkoxide precursor (tetraethyl orthosilicate (TEOS)), and a surfactant (cetyltrimethylammonium bromide (CTAB)). This method consists of the formation of mesoporous nanoparticles under the size range of 60 nm to 1000 nm. The particles were dried at room temperature and calcined at 550°C for 3 h. Subsequently, HP solution was placed adjacent to synthesized nanoparticles. The HP enters into the MSN cavities passively process (23, 24).
3.2. Tumor Model
To use a syngeneic tumor model, the confirmed murine spontaneous breast adenocarcinoma was got rid of from anesthetized Balb/C mice (ketamine/xylazine, 30 mg/kg, IP). The tumor tissue was chopped into fresh pieces with a diameter of 2 mm to 3 mm in PBS. A portion of the tumor was subcutaneously placed in the inguinal area of the receptor animal (Inbred Balb/C female mice, 6 - 8 week-old), and suture clips were used to close the incision. To prevent mice infection, Cefazolin (200 mg/Kg) was dissolved in the animals’ water (25). All procedures were approved by the Research Ethics Committee of Semnan University of Medical Sciences (IR.SEMUMS.REC.1396.18), which was following the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals (NIH Publications No. 8023, revised Edition 1978).
3.3. Ultrasound/Laser Radiation
For ultrasound/laser radiation, the mice were anesthetized, using intraperitoneal ketamine and xylazine. Anesthetized mice with grafted tumors were placed moveless by a specific holder in the near field of ultrasonic waves (30 cm) in a cubic Plexiglas water tank (25 × 25 × 35 cm3). Two ultrasonic probes (5 cm diameter) were fixed in a 90° position and the central beam of each ultrasound wave was at right angles to the axis of the other. The first source was a 1 MHz (1, 2 W/cm2) and the other source was a 3 MHz (1, 2 W/cm2) ultrasonic treatment system (210P and 215A, Novin Medical Engineering, Isfahan, Iran). The animals were exposed to 150 mW of 650 nm laser light simultaneously with ultrasound, and the time of the ultrasound/laser process was 60 seconds.
3.4. Treatment Groups and Tumor Evaluation
The treatment method started when the average diameter of tumors reached 7 mm to 10 mm. To assess the effect of dual-frequency SPDT with injection of sensitizer on breast adenocarcinoma, 132 tumor-bearing female mice were separated randomly into 22 groups (n = 6): sham (solvent injection), laser, 4 groups of dual-frequency ultrasound and laser radiation: 1, 3 MHz (1 and 2 W/cm2) + laser, 8 groups of HP-mediated SPDT: 1, 3 MHz (1 and 2 W/cm2) + HP (2.5 and 5 mg/kg) + laser, and 8 groups of HP-MSNs-mediated SPDT: 1, 3 MHz (1 and 2 W/cm2) + HP-MSNs (2.5 and 5 mg/kg) + laser. Due to the weight of experimental mice (20 ± 2 g), a dose of 10 mg/kg (0.2 mL) HP, or HP-MSNs were injected intra-peritoneal 24 h before ultrasound (26).
After SPDT performance, to evaluate the tumor volume, the length (a), width (b), and depth (c) of each tumor was measured with a digital caliper every 3 days and mass volume was estimated from the volume formula: V = 0.5 × a × b × c. The calculated volumes (V) were used to evaluate tumor growth parameters: Relative volume (Relative volume = [(V-Vo)/Vo] × 100), tumor growth inhibition ratio (TGI% = [1- (Vxday /Vcotrolday)] × 100), and the times needed for each tumor to reach 2 (T2) and 5 times (T5) to the primary mass volume (26). Histopathological images of mass sections were obtained 30 days after the treatment. Tumor sections were stained with hematoxylin/eosin to assess tumor grading and malignancy based on Bloom-Richardson (BR) classification (tumor tubule formation, the number of mitosis/10 high power fields, and nuclear grade). The degree of tumor grading was low grade (well-differentiated), intermediated grade (moderately-differentiated), and high grade (poorly-differentiated) (27). The histopathological analysis was performed blindly.
3.5. Analysis
Statistical analysis was performed, using SPSS 24.0 (SPSS/PC Inc., Chicago, IL, USA). Summary statistics for all normally distributed variables are presented as the mean ± standard deviation for each group. One-way analysis of variance (ANOVA) was used for comparison of the mean of independent groups. For multiple comparisons, after one-way ANOVA, we used the Tukey test. Survival analysis was carried out with the Kaplan-Meier and log-rank test for investigating survival times.
4. Results
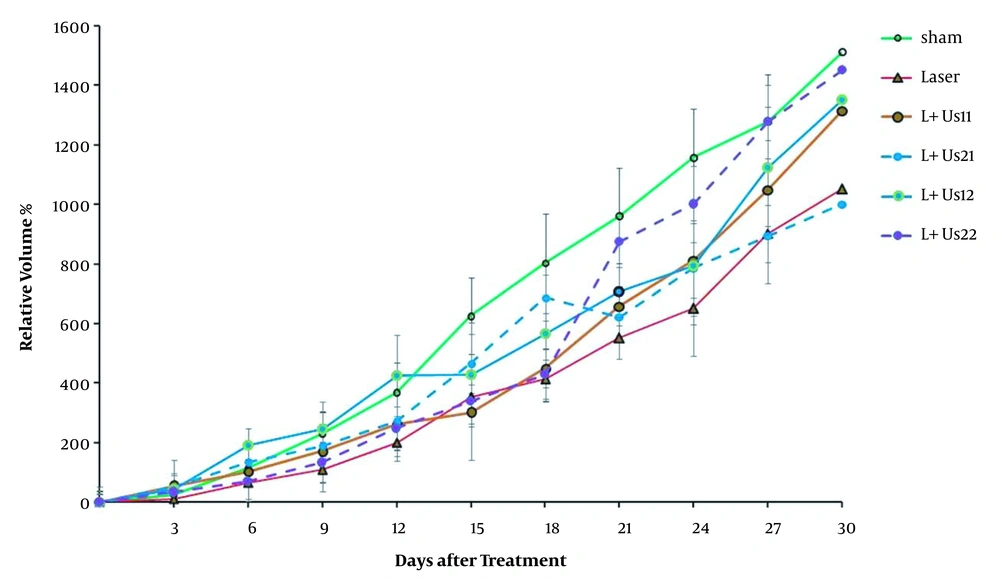
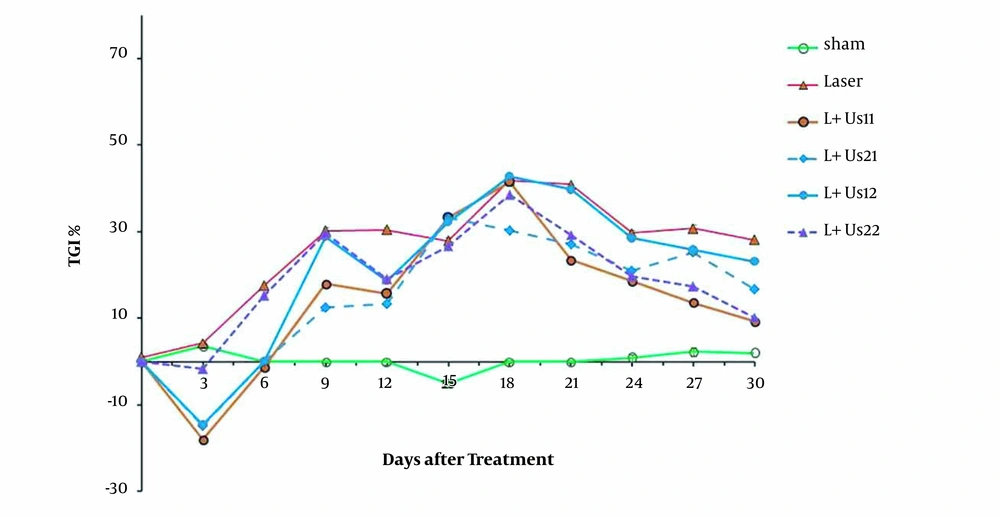
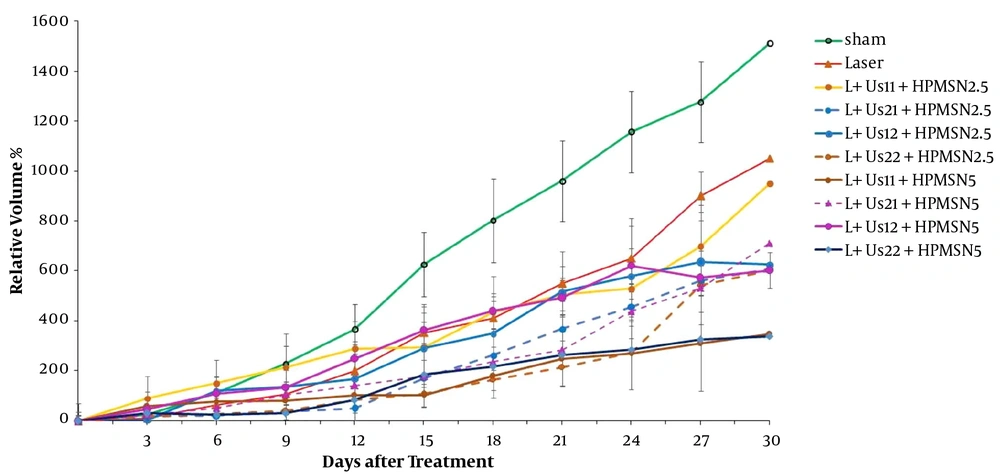
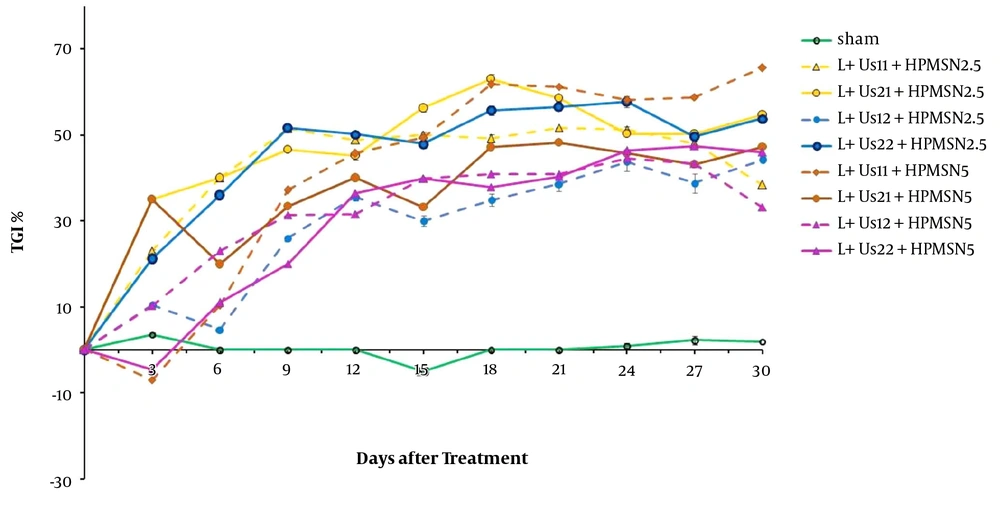
The results obtained from the relative tumor volume after treatment with dual-frequency ultrasound and laser radiation have been plotted in Figure 1. These results indicate that ultrasound and laser radiation have delayed effective tumor growth in comparison with the control and sham groups at 18 days after the treatment. Analysis of data showed non-significant differences between groups before 18 days (P > 0.05). Comparison of data showed non-significant differences between groups to reach 2 times and 5 times primary volume in the presence of irradiation (P > 0.05). The required time of T2 to the initial volume in groups of dual-frequency ultrasound with 3 MHz (2 W/cm2) radiation was greater than other groups (8 and 6 days, respectively). Analysis of T5 data showed non-significant differences between groups (P > 0.05). The inhibition of tumor growth (TGI%) over the experimental period was shown in Figure 2. TGI in the groups that behaved with dual-frequency ultrasound and laser radiation was higher than that of the sham group. The maximum TGI ratio was shown at 18 days after the treatment. The result of the experiment demonstrates that this increase was transient and declined over 30 days of treatment.
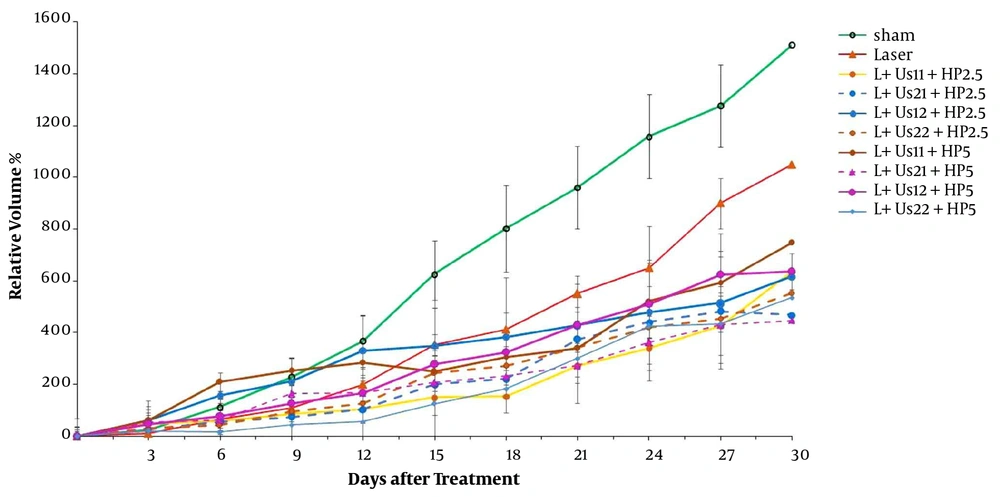
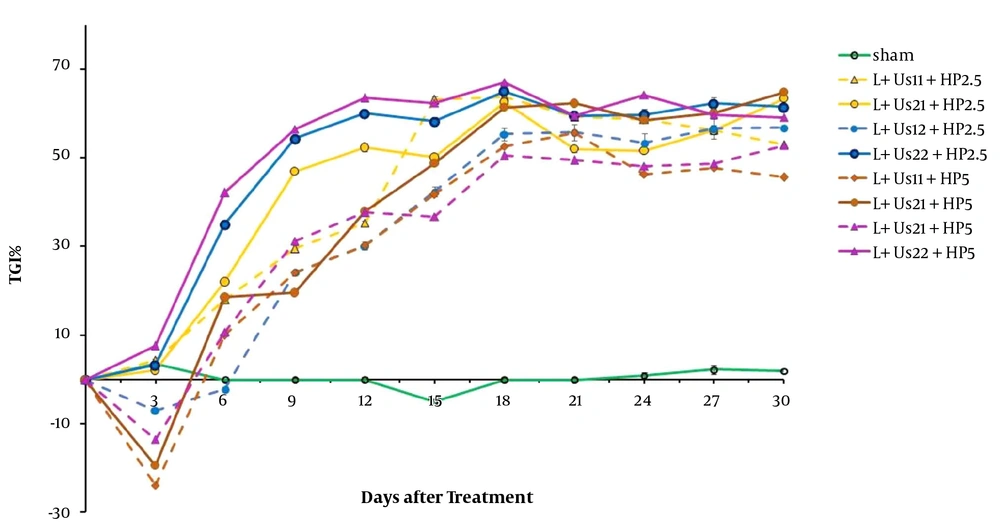
To validate our findings, we estimated the anti-tumor effects of HP-mediated SPDT. Figure 3 demonstrates the post-treatment relative tumor volume. Significant differences were detected between experimental groups and sham in tumor volume 15 days after the treatment (P < 0.05). Comparison of data showed a non-significant difference between HP-mediated SPDT groups (P > 0.05). Investigation of T2 data showed a non-significant difference between HP-mediated SPDT groups (P > 0.05). The required time of T5 to the initial volume in groups of 5 mg/kg HP injected was higher than the sham and HP 2.5 mg/kg groups (18, 14, and 15 days, respectively). As presented in Figure 4, the percent of TGI in the groups of HP-mediated SPDT was greater than that of the sham group. The maximum TGI ratio was shown at 12 days after the treatment. The experiment demonstrates that this increase was not temporary and persisted over 30 days of treatment.
Figure 5 demonstrates tumor growth curves over 30 days after the treatment. These results indicate that HP-MSNs-mediated SPDT is effective in delaying tumor growth when compared with the sham group (P < 0.05). Overall comparison of data showed a non-significant difference between SPDT with HP-MSN (2.5 and 5 mg/kg) groups (P > 0.05). The time of T2 in the case of HP-MSNs-mediated SPDT groups increased in comparison with sham and that of HP-mediated SPDT groups (13, 6, and 9 days, respectively). In addition, the required time of T5 to the initial volume in all HP-MSNs-mediated SPDT groups was higher than that in the sham and HP-mediated SPDT groups (P < 0.05). Furthermore, the T5 time in the trial of dual-frequency SPDT (3 MHz, 2 W/cm2) with HP-MSN (5 mg/kg) group rose to 27 days. Figure 6 represents mice breast adenocarcinoma TGI% over 30 days of treatment. TGI in the groups of HP-MSNs-mediated SPDT was higher than that of the sham group (P < 0.05). In the groups treated with dual-frequency ultrasound and laser radiation, the maximum TGI ratio was 47.5%, while the maximum TGI ratio in the SPDT groups was 61.6%. The TGI ratio increased in all groups at 12 days after the treatment. Analysis of experimental data demonstrates that this increase was not declined and persisted over 30 days of treatment. The results indicated that HP-mediated and HP-MSNs-mediated SPDT are effective in relative tumor volume when compared with the sham group (447.7 ± 119, 339.1 ± 161, and 1510.8 ± 160, respectively).
Kaplan-Meier analysis of experimental data revealed that the 72 days survival (cumulative survival fraction) was 95% for the 5 mg/kg HP-MSNs-mediated SPDT group. The survival meantime (with 95% confidence interval) for the sham, laser, dual-frequency ultrasound, HP-mediated SPDT (5 mg/kg) groups was 32, 34, 35, and 51 days, respectively; the overall comparison test of survival equality for the different levels of groups demonstrated a significant difference between experimental groups: Log Rank (Mantel-Cox), P = 0.014.
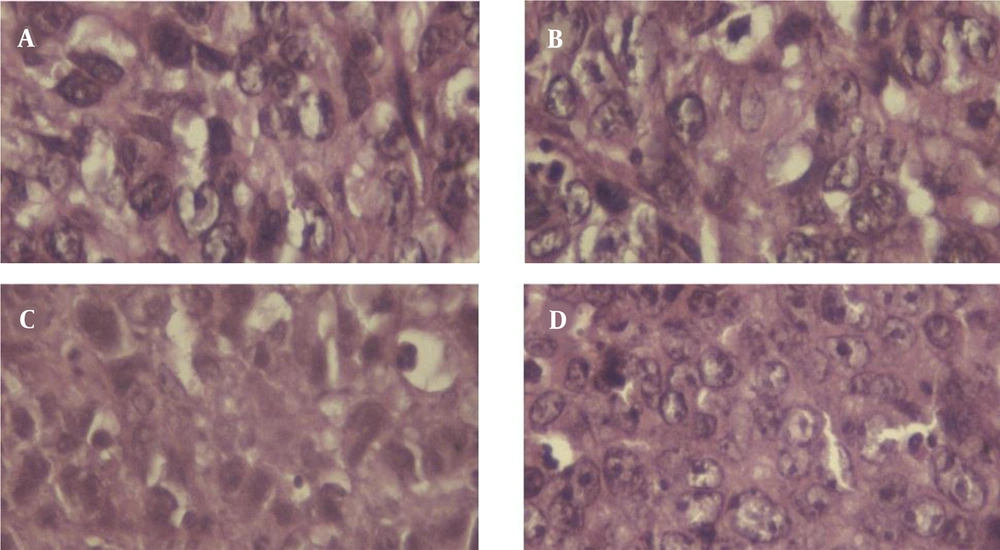
Microscopically assessment of tumor sections revealed multiple nuclear mitosis and polymorphism in all investigational groups (Figure 7). The results of the histopathological study to determine the grading of the tumor were shown in Table 1. The sham and laser groups had grade III malignancy (poorly differentiated), while HP-MSNs-mediated SPDT groups had grade II malignancy (well-differentiated) in the histological study of mice breast adenocarcinoma.
| Group | Tumor Tubule Formation | Number of Mitosis | Total Score | BR Grade | BR Grade | Grade |
|---|---|---|---|---|---|---|
| Sham | 3 | 3 | 3 | 9 | Poorly differentiated | 3 |
| Laser | 3 | 3 | 3 | 9 | Poorly differentiated | 3 |
| Laser + US22 | 1 | 3 | 3 | 7 | Moderately differentiated | 1 |
| Laser + US22 + HP5 | 2 | 2 | 2 | 5 | Well differentiated | 1 |
| Laser + US22 + HPMSN5 | 1 | 2 | 1 | 4 | Well differentiated | 1 |
5. Discussion
The results of the above-mentioned experiments show that the combined dual-frequency ultrasound (1 + 3 MHz) and/or laser (650 nm) radiation caused a transient inhibition effect on mice breast adenocarcinoma tumor growth. Also, HP-mediated SPDT (2.5 and 5 mg/kg) caused anti-tumor effect. Moreover, HP-MSNs-mediated SPDT (2.5 and 5 mg/kg) had inhibition effect on tumor growth. The TGI ratio increased in all experimental groups at 12 days after the initiation of radiation and persisted over 30 days of treatment. These findings are in agreement with Barati et al.'s findings that dual ultrasound (1 MHz + 150 kHz) for 30 min decreased mice breast adenocarcinoma tumor growth (28). In addition, Guan and Xu showed that high-intensity focused ultrasound (1.6 MHz) could destroy proliferating tumor cells in human breast cancer (29). In agreement with our results, the findings of Banerjee et al. confirm a potential role for PDT with verteporfin and laser (690 nm) in the management of early breast cancer in 12 female patients (1). Miyoshi et al. suggested that combination therapy of SDT (titanium oxide)/ PDT (aminolevulinic acid) can help to get a reasonable anti-tumor effect on mice squamous cell carcinoma because the ultrasound (1 MHz) deeper penetration into the cancer tissue was compared with the laser light (635 nm) (5). Moreover, the findings demonstrated that chlorin e6-mediated SPDT enhanced the antitumor efficacy on 4T1 mammary cancer cells compared with SDT (1 MHz) and PDT (laser 650 nm) alone (30). The combination of PDT (665 nm) and SDT (3.3 MHz) have shown an improved glioblastoma cell in vitro and in vivo, which could be referred to as a synergetic effect. Exposing the nano-formulation HPPH with ultrasound also triggered the release of PS (31). In An et al.'s study, a 630 nm semiconductor laser and 1 MHz ultrasound were used to perform SPDT. In An et al.'s study, SPDT with 630 nm laser and 1 MHz ultrasound + sinoporphyrin sodium inhibited glioma cell proliferation and induced cell apoptosis due to the generation of ROS and affecting protein expression (32). Moreover, Hong et al. proposed SPDT with a nanoformulation Ce6-P/WNE in the treatment of prostate cancer cells. The results concluded that activated Ce6-P/WNEs in tumor cells by light (633 nm) and/or ultrasound (2.1 MHz) produced ROS even in a hypoxic environment (19).
The structure of mesoporous channels would allow controllable drug release by mechanical and cavitation effects of ultrasound (33). The collapse of cavitating bubbles can cause sonomechanical and sonochemical cytotoxic effects and the formation of cytotoxic reactive oxygen species (34). Hence, this means that a combination of ultrasound and HP-MSNs could have a better treatment effect on mice breast adenocarcinoma. In agreement with our findings, Zhang et al. investigated that the therapeutic effect of encapsulated HP-SDT was better than HP or ultrasound alone (35). In addition, during a study by Hasanzadeh et al. to review the effect of dual-frequency ultrasound radiation on nanomicellar containing doxorubicin, the SDT increased the ultrasound cavitations' efficiency (13).
Cellular antioxidant capacity reduction may cause by oxidative stress. Free radical formation impairs the cell membrane fatty acids and proteins' function. As well, free radical production reacts with gene mutation and DNA damage, which provokes cancer development (36). PDT applies its effects when the light is used to activate a photochemical reaction of a non-toxic PS, which generates reactive oxygen species (ROS). These ROS cause oxidative damage to lipids, proteins, and nucleic acids, which lead to the destruction of cancer cells through apoptosis or necrosis. The mentioned constructions have strong light absorption at wavelengths > 650 nm and are essential for good tissue penetration of light (37). The destruction effect of PDT not only depends on the type of PS, but also is related to light exposure and fluency, oxygen level, localization of sensitizer, drug administration time, and other parameters (38). Despite the satisfactory advantages of PDT, the clinical application of this approach has been limited. Several reasons can be explained such as the poor penetration of light and its dependence on the tumor tissue oxygen presence (39).
Ultrasound good tissue penetration and wave energy focusing on the depth of tumor tissue can produce bio-effects (11). It mentioned that acoustic cavitation is the main cause of destructive chemical reactions and the free radical production since ultrasound irradiation. During a study by Feng et al., the effect of mixing two-frequency and three-frequency ultrasonic waves on cavitations' efficiency was investigated. The results showed that irradiation of two or more ultrasound waves significantly increases the cavitations' efficiency compared to single-frequency irradiation (40). The simultaneous effects of sensitizer and ultrasound comprise mechanical, chemical, and cavitational mechanisms (28). Protoporphyrin that is used in PDT can generate active oxygen species after activation by visible light (41). SDT followed by PDT can help to get a realistic anti-tumor effect because ultrasound has deeper penetration into the tumor tissue compared to laser light. Besides the singlet oxygen mechanism in PDT, mechanical stress, cavitational effects, and reactive oxygen species comprise SDT (4).
The histopathological results (Bloom-Richardson classification basis) showed that the sham and laser groups had grade III malignancy (poorly differentiated), while HP-mediated SPDT and HP-MSNs-mediated SPDT groups had grade I malignancy (well-differentiated) in the histological study of mice breast adenocarcinoma. Overall comparison test of survival equality for the different levels of groups demonstrated a significant difference between groups: Log rank (Mantel-Cox), P = 0.014. This may cause simultaneous radiation of dual-frequency ultrasound and laser radiation. On contrary, analysis of the author's previous investigation revealed that injection of HP and HP-MSNs (2.5 mg/kg) did not show any effect on T2 time and tumor relative volume, and the tumors had a poorly differentiated grading. The results of single-frequency SDT were not only determined by ultrasound wave power density, but also were related to HP-MSN injected dose (25). Hong et al.'s study demonstrated that to overcome the limitations of each modality in the hypoxia environment, the strengths of PDT and SDT could be combined. The ultrasound deep tissue penetration combined with sensitizer activation by light will significantly enhance the therapeutic applications for prostate cancer cells (19). In theory, the tumor cell toxicity effects of SDT and PDT are facilitated by cytotoxic drugs that are produced by sonochemical or photochemical reactions (42).
The opinion of SPDT is based on the special buildup of sensitizer in tumors, and the improved cytotoxicity after activation by ultrasound or light. The imaginable tumor uptake could be explained as (1) selective buildup related to the tumor surrounding microenvironment, (2) lipoprotein receptors in some of the tumor cells, and (3) endocytosis mechanism to the entrance of sensitizer and low-density protein binding. In addition, because of the lower pH value in tumors, tumor-associated macrophages receive large amounts of porphyrin derivatives (4). SPDT has been used in the treatment of many cancers with variable success, but the efficacy of breast adenocarcinoma damage induced by dual-frequency SPDT with HP-MSNs has rarely been reported. The novelty of the present SPDT study was activation of HP and HP-MSNs (2.5 and 5 mg/kg) in the presence of two intensities (1 and 2 W/cm2) of 1 and 3 MHz ultrasound and laser radiation (650 nm) in the management of Inbred Balb/C mice grafted breast adenocarcinoma. Aside from only one laser light radiation (650 nm), another limitation of this research was once sensitizer injection with a temporary treatment effect. The fractional injection of sensitizer should be done in our future study.
5.1. Conclusions
The results of this study demonstrated that HP and HP-MSNs-mediated SPDT have an anti-tumor effect in mice breast adenocarcinoma. It can be appreciated that careful selection of the sensitizer with SPDT will play an eminent role in the success of cancer therapies. Improvement sensitizer agents used in PDT and SDT are useful drugs for SPDT and can expand cancer treatment management. However, further studies are required to optimize the sensitizer, light/laser, and ultrasound parameters to find better tumor treatment methods and explain the mechanism of SPDT.