1. Background
Colorectal cancer (CRC) is the third most common cancer (10.2%) and the second leading cause of cancer death (9.2%) worldwide according to GLOBOCAN 2018. About 20% of patients with colon cancer are diagnosed with synchronous metastatic disease, most of which occurs in the liver (1). Although patients with colorectal liver metastases might be considered for curative treatment, the median overall survival of these patients is still poor (2). Treatment of patients with colorectal liver metastases is selected based on the, resectability of the lesions. Only a few of them are considered appropriate for liver resection (3). Systemic chemotherapy with or without targeted therapy is increasingly used for both resectable and unresectable diseases. Unfortunately, the response rate to first-line chemotherapy in metastatic colorectal cancer (MCRC) is only 50% (4). Finding markers to predict response to chemotherapy is a critical issue. Most studies on predicting response to systemic therapy in CRC are focused on molecular predictive factors like microsatellite instability, RAS, and BRAF, which are mostly expensive and not readily available in developing countries like Iran. In this regard, finding inexpensive and readily available markers to predict response to the systemic agent would be of value to distinguish the responders from non-responders and guide the treatment approach of MCRC.
The Association of inflammation and cancer has been first described in the 19th century (5). It has been proved that high levels of inflammatory markers are associated with poor outcomes in patients with cancer, especially CRC (6). Complete blood count (CBC) test might be one of the most common and available types of laboratory studies. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR), which can be easily calculated based on pretreatment CBC, have been used to define the inflammatory state of the patients (7, 8). Currently available pieces of evidence have shown that the high level of NLR and PLR are poor prognostic factors for several types of cancers like lung cancer, esophageal cancer, gastric cancer, breast cancer, and CRC (9, 10). Data on whether NLR and PLR could be used as a marker to predict response to chemotherapy in CRC patients with synchronous liver metastasis are scares and these markers are not used for decision making in daily practice.
2. Objectives
This study aimed at answering whether these two inexpensive markers could be used to predict clinical response to first-line chemotherapy in CRC with synchronous liver metastases.
3. Methods
3.1. Patients
This retrospective study was carried out based on hospital records of patients with CRC, who were treated at two tertiary referral hospitals (Imam Reza Hospital and Imam Khomeini Hospital Complex) from January 2015 to August 2020 in Tehran, Iran. Patients with synchronous liver metastases confirmed by pathology (with or without lung metastases), who underwent upfront systemic therapy, were identified. All patients received first-line chemotherapy including FOLFOX (folinic acid, fluorouracil, oxaliplatin), FOLFIRI (folinic acid, fluorouracil, irinotecan), or CAPOX (capecitabine, oxaliplatin) with or without target therapy. Patients who underwent primary liver resection before chemotherapy, those with a history of another malignancy, and/or previous cytotoxic therapy were excluded. Baseline clinicopathologic findings of the patients including age, sex, performance status, histologic differentiation of the specimen, primary tumor site, IHC reports (i.e. KRAS, NRAS, and BRAF), serum carcinoembryonic antigen (CEA), and albumin were obtained. Performance status was defined based on the Eastern Cooperative Cancer Group (ECOG) scale of Performance Status. The primary tumor site was recorded as right colon (cecum, ascending colon, and transverse colon) or left colon (splenic flexure, descending colon, recto-sigmoid).
3.2. NLR and PLR Level
NLR was defined by dividing absolute neutrophil count to absolute lymphocyte count and PLR was defined by dividing absolute platelet count to absolute lymphocyte count in the blood sample, which was taken within 72 hours of the first cycle of chemotherapy. Patients with signs and symptoms of infection at the time of blood sampling were excluded. Groups were defined based on the level of NLR and PLR as high NLR or low NLR and high PLR or low PLR.
3.3. Assessment of Response to Chemotherapy
Response evaluation was done by computed tomography (CT) scans of the chest, abdomen, and pelvic. A baseline CT scan was carried out within 2 weeks before chemotherapy. A Follow-up CT scan was done after 8 weeks of chemotherapy. To avoid inter-observer bias, only patients, whose pretreatment and follow-up CT scans were compared and reported by the same radiologist according to Response Evaluation Criteria in Solid Tumors criteria (RECIST1.1), were included in the final analysis (11). Objective response (OR) was defined as a complete response or partial response. Different clinicopathologic variables, including NLR and PLR, were compared in patients with or without OR. The study was approved by the institutional ethics committee.
3.4. Statistical Analysis
Continuous data were expressed as mean ± standard deviation (SD) and categorical data were presented by frequency and percentage. Continuous and categorical variables were compared, using the t-test and the chi-square or Fisher exact test, respectively. The appropriate cut-off for NLR and PLR was defined by calculating the maximum Youden index (YI) according to the receiver operating characteristic (ROC) curve for predicting objective response. Logistic regression analysis was performed to determine which variables are independently associated with an objective response. For all statistical tests, we defined 0.05 as the significance level. Statistical analysis was done by SPSS software version 26.0.
4. Results
4.1. Patient’s Characteristics
From January 2015 to August 2020, hospital records of 116 CRC patients with synchronous metastases to the liver, who underwent upfront chemotherapy, were identified. After the exclusion of the patients with unavailable NLR and PLR data (n = 14) and the ones who did not have radiological reports for response evaluation based on RECIST criteria (n = 21), 81 were finally included in the analysis. Fifty-three (65.4%) patients were male and 28 (36.6%) were female. The mean age of the patients was 54.84 ± 10.29 years old. The primary tumor was in the right colon in 34 (42%) and left colon in 47 (58%) patients. FOLFOX regimen was the most common regimen used for first-line chemotherapy (66.7%) and bevacizumab was the most common targeted therapy (60.4%). The overall mean value of NLR and PLR was 3.62 ± 2.82 and 185.07 ± 95.87 respectively.
4.2. The Optimal Cut-off for NLR and PLR
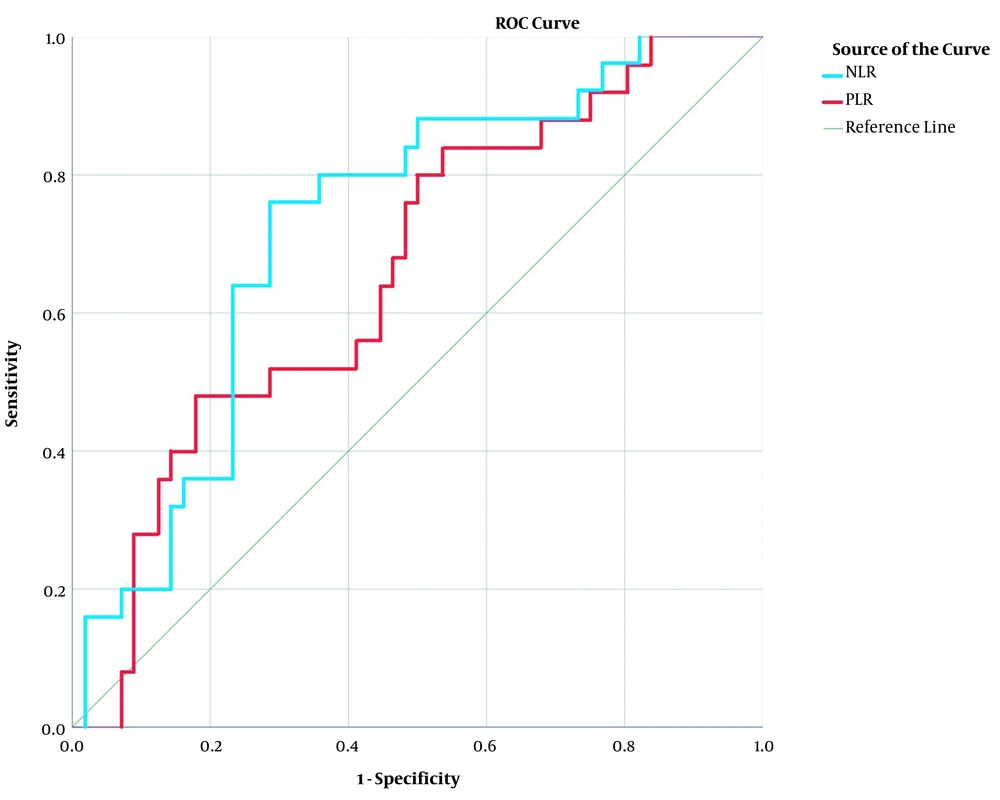
To define the optimal cut-off for NLR and PLR, we generated a ROC curve to calculate the area under the curve (AUC). AUC for objective response for NLR and PLR was 0.725 (95% CI, 0.539 - 0.732; P = 0.001) and 0.661 (95% CI, 0.450 - 0.715; P = 0.021), respectively (Figure 1). The maximum Youden index was 0.474 when NLR was 2.666; so, patients were grouped as low NLR and high NLR if the ratio was < 2.666 or ≥ 2.666, respectively. Youden index was at its maximum (0.304) when PLR was 182.589; so, patients with PLR < 182.589 and ≥ 182.589 were grouped as low PLR and high PLR, respectively.
ROC curve for NLR and PLR. The ROC curve for NLR with an AUC of 0.725 (95% CI, 0.539 - 0.732; P = 0.001) is indicated by blue line. The ROC curve for PLR with an AUC of 0.661 (95%CI, 0.450 - 0.715; P = 0.021) is indicated by red line. NLR, neutrophil lymphocyte ratio; PLR, platelet lymphocyte ratio; ROC, receiver operating characteristic; AUC, area under the ROC curve; and CI, confidence interval.
4.3. Patient’s Characteristics Based on NLR and PLR
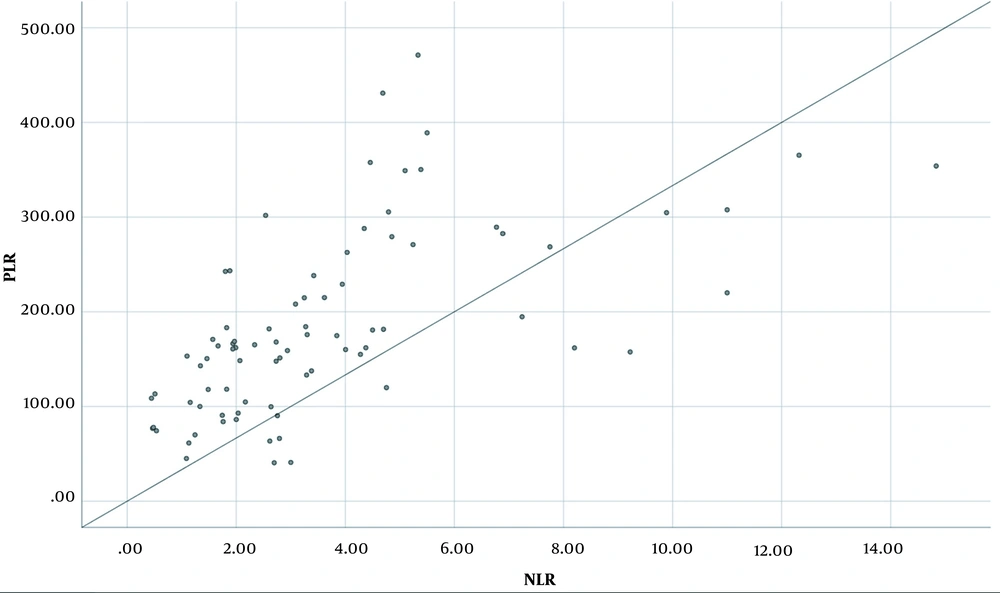
Baseline characteristics of the patients based on NLR and PLR are shown in Table 1; 35 (43.2%) patients were grouped as low NLR and 46 (56.8%) as high NLR, while 51 (63%) patients were grouped as low PLR and 30 (37%) as high PLR. Serum albumin level was significantly lower in the high NLR group and high PLR group compared to the low NLR group (P = 0.009) and low PLR group (P = 0.022), respectively. No other significant difference was seen based on NLR and PLR. A positive association between NLR and PLR was seen; the regression equation was y = 20.614x +108.99 (adjusted R2 = 0.361; P < 0.000) (Figure 2).
| Characteristics | NLR | PLR | ||||
|---|---|---|---|---|---|---|
| Low NLR (n = 35) | High NLR (n = 46) | P-Value | Low PLR (n = 51) | High PLR (n = 30) | P-Value | |
| Age (y) | 53.69 ± 10.67 | 55.72 ± 10.02 | 0.38 | 54.92 ± 10.51 | 54.70 ± 10.08 | 0.92 |
| Gender | 0.48 | 0.81 | ||||
| Male | 21 (60.0) | 32 (69.6) | 34 (66.7) | 19 (63.3) | ||
| Female | 14 (40.0) | 14 (30.4) | 17 (33.3) | 11 (36.7) | ||
| PS | 0.50 | 0.35 | ||||
| 0 | 13 (37.1) | 21 (45.7) | 19 (37.3) | 15 (50) | ||
| 1 - 2 | 22 (62.9) | 25 (54.3) | 32 (62.7) | 15 (50) | ||
| Laterality | 0.11 | > 0.9 | ||||
| Right colon | 11 (31.4) | 23 (50.0) | 21 (41.2) | 13 (43.3) | ||
| Left colon | 24 (68.6) | 23 (50.0) | 30 (58.8) | 17 (56.7) | ||
| Site of metastases | 0.80 | 0.31 | ||||
| Liver only | 25 (71.4) | 34 (73.9) | 35 (68.6) | 24 (80.0) | ||
| Liver and lung | 10 (28.6) | 12 (26.1) | 16 (31.4) | 6 (20.0) | ||
| Differentiation | 0.12 | 0.26 | ||||
| Well and moderate | 28 (80.0) | 27 (58.7) | 38 (74.5) | 17 (56.7) | ||
| Poor | 5 (14.3) | 13 (28.3) | 8 (15.7) | 10 (33.3) | ||
| Unknown | 2 (5.7) | 6 (13.0) | 5 (9.8) | 3 (10.0) | ||
| KRAS | 0.86 | 0.40 | ||||
| Wild type | 20 (57.1) | 27 (58.7) | 27 (52.9) | 20 (66.7) | ||
| Mutant | 10 (28.6) | 11 (23.9) | 14 (27.5) | 7 (23.3) | ||
| Unknown | 5 (14.3) | 8 (17.4) | 10 (19.6) | 3 (10) | ||
| Obstruction | 0.73 | > 0.9 | ||||
| No | 30 (85.7) | 41 (89.1) | 45 (88.2) | 26 (86.7) | ||
| Yes | 5 (14.3) | 5 (10.9) | 6 (11.8) | 4 (13.3) | ||
| Primary resection | 0.14 | 0.45 | ||||
| No | 22 (62.9) | 35 (76.1) | 34 (66.7) | 23 (76.7) | ||
| Yes | 13 (37.1) | 11 (23.9) | 17 (33.3) | 7 (23.3) | ||
| Size of primary tumor (cm) | 4.68 ± 1.32 | 4.22 ± 1.29 | 0.33 | 4.53 ± 1.30 | 4.33 ± 1.39 | 0.71 |
| Chemotherapy | 0.68 | 0.11 | ||||
| FOLFOX | 25 (71.4) | 29 (63.0) | 37 (72.5) | 17 (56.7) | ||
| FOLFIRI | 7 (20.0) | 13 (28.3) | 12 ( 23.5) | 8 (26.7) | ||
| CAPOX | 3 (8.6) | 4 (8.7) | 2 (3.9) | 5 (16.7) | ||
| Target therapy | 0.85 | 0.81 | ||||
| Bevacizumab | 22 (62.9) | 27 (58.7) | 32 (62.7) | 17 (56.7) | ||
| Cetuximab | 8 (22.9) | 13 (28.3) | 12 (23.5) | 9 (30.0) | ||
| None | 5 (14.3) | 6 (13.0) | 7 (13.7) | 4 (13.3) | ||
| Albumin (g/dL) | 4.26 ± 0.67 | 3.65 ± 0.61 | 0.009 | 4.17± 0.64 | 3.50 ± 0.68 | 0.022 |
| CEA (ng/mL) | 252.58 ± 526.52 | 1151.52 ± 3952.02 | 0.30 | 222.85 ± 375.46 | 1301.09 ± 3815 | 0.27 |
Abbreviations: NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; PS, performance status, FOLFOX, folinic acid, fluorouracil, oxaliplatin; FOLFIRI, folinic acid, fluorouracil, oxaliplatin; CAPOX, capecitabine, oxaliplatin; and CEA, carcinoembryonic antigen.
aValues are expressed as mean ± SD and No. (%).
4.4. Response to Chemotherapy
The response was defined based on the RECIST criteria. The overall number of patients with complete response, partial response, stable disease, and progressive disease was 4 (4.9%), 21 (25.9%), 36 (44.5%), and 20 (24.7%), respectively; 25 out of 81 patients (30.86%) objectively responded to first-line chemotherapy regimens.
Response to chemotherapy in patients, based on NLR and PLR, is shown in Table 2. Patients with low NLR had a significantly higher objective response rate compared to patients with high NLR (54.3% versus 13% respectively, P < 0.001); 41.2% of patients with low PLR had objective response compared to 13.3% of patients with high PLR (P = 0.012). On the other hand, 37% of patients with high NLR and 40% of patients with high PLR progressed during first-line chemotherapy.
| Response | NLR | PLR | ||||
|---|---|---|---|---|---|---|
| Low NLR (n = 35) | High NLR (n = 46) | P-Value | Low PLR (n = 51) | High PLR (n = 30) | P-Value | |
| CR | 3 (8.6) | 1 (2.1) | < 0.001 | 4 (7.8) | 0 (0.0) | 0.02 |
| PR | 16 (45.7) | 5 (10.9) | 17 (33.3) | 4 (13.3) | ||
| SD | 13 (37.1) | 23 (50.0) | 22 (43.1) | 14 (46.7) | ||
| PD | 3 (8.6) | 17 (37.0) | 8 (15.7) | 12 (40) | ||
| OR | 19 (54.3) | 6 (13) | < 0.001 | 21 (41.2) | 4 (13.3) | 0.012 |
Abbreviations: NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio, CR, complete response, PR, partial response, SD, stable disease, PD, progressive disease, and OR, Objective response.
aValues are expressed as No. (%).
Objective response was not associated with any of the primary clinicopathologic features other than NLR and PLR (Table 3). The mean ± SD of NLR in 25 patients with objective response was 2.30 ± 1.50 compared to 4.30 ± 3.05 in those without objective response (P < 0.001). The mean PLR in patients with objective response was 146.41 ± 70.21 compared to 202.34 ± 101.05 in those without objective response (P = 0.006). The univariate analysis determined that both NLR and PLR are significantly associated with the objective response, but in multivariate analysis, only NLR was identified as a significant and independent predictive marker of response (Table 4).
| Characteristics | Objective Response | P-Value | |
|---|---|---|---|
| No (n = 56) | Yes (n = 25) | ||
| Age (y) | 55.41 ± 9.84 | 53.56 ± 11.34 | 0.48 |
| Gender | > 0.9 | ||
| Male | 37 (66.1) | 16 (64) | |
| Female | 19 (33.9) | 9 (36) | |
| PS | 0.49 | ||
| 0 | 23 (41.1) | 11 (44) | |
| 1 - 2 | 33 (58.9) | 14 (56) | |
| Laterality | 0.62 | ||
| Right colon | 25 (44.6) | 9 (36) | |
| Left colon | 31 (55.4) | 16 (64) | |
| Site of metastases | 0.42 | ||
| Liver only | 39 (69.6) | 20 (80) | |
| Liver and lung | 17 (30.4) | 5 (20) | |
| Differentiation | 0.86 | ||
| Well and moderate | 37 (66.1) | 18 (72) | |
| Poor | 13(23.2) | 5 (20) | |
| Unknown | 6 (10.7) | 2 (8) | |
| KRAS | 0.96 | ||
| Wild type | 32(57.1) | 15 (60) | |
| Mutant | 15 (26.8) | 6 (24) | |
| Unknown | 9 (16.1) | 4(6) | |
| Obstruction | 0.34 | ||
| No | 48 (85.7) | 23 (92) | |
| Yes | 8 (14.3) | 2 (8) | |
| Primary resection | 0.47 | ||
| No | 40 (71.4) | 17 (68) | |
| Yes | 16 (28.6) | 8 (32) | |
| Size of primary tumor (cm) | 4.40 ± 1.19 | 4.76 ± 1.81 | 0.33 |
| Chemotherapy | 0.42 | ||
| FOLFOX | 35 (62.5) | 19 (76) | |
| FOLFIRI | 15 (26.8) | 5 (20) | |
| CAPOX | 6 (10.7) | 1 (4) | |
| Target therapy | 0.90 | ||
| Bevacizumab | 34 (60.7) | 15 (60) | |
| Cetuximab | 15 (26.8) | 6 (24) | |
| None | 7 (12.5) | 4 (16) | |
| Albumin (g/dL) | 4.01 ± .69 | 3.96 ± 0.76 | 0.85 |
| CEA (ng/mL) | 982.73 ± 3198.21 | 205.46 ± 327.20 | 0.26 |
| NLR | 4.30 ± 3.05 | 2.30 ± 1.50 | < 0.001 |
| PLR | 202.34 ± 101.05 | 146.41 ± 70.21 | 0.006 |
Abbreviations: NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; PS, performance status, FOLFOX, folinic acid, fluorouracil, oxaliplatin; FOLFIRI, folinic acid, fluorouracil, oxaliplatin; CAPOX, capecitabine, oxaliplatin; and CEA, carcinoembryonic antigen.
aValues are expressed as mean ± SD and No. (%).
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (CI 95%) | P-Value | OR (CI 95%) | P-Value | |
| NLR (low/high) | 7.91 (2.67 - 23.4) | < 0.001 | 5.99 (1.84 - 19.45) | 0.003 |
| PLR (low/high) | 4.55 (1.38 - 14.7) | 0.013 | 2.05 (0.53 - 7.94) | 0.29 |
Abbreviations: NLR: neutrophil to lymphocyte ratio; PLR: platelet to lymphocyte ratio; OR: odds ratio; CI: confidence interval.
5. Discussion
High NLR and PLR have been linked to worse prognoses in different cancers, especially CRC. To the best of our knowledge, a small number of studies have assessed the significance of NLR and PLR in predicting clinical response to chemotherapy. Whether these markers can be used to distinguish responders from non-responders in MCRC is unclear. In the present study, we evaluated and compared the role of NLR and PLR in predicting response to first-line chemotherapy in treatment-naive MCRC. Although the high level of both NLR and PLR are significantly associated with the lower response rate in CRC with synchronous liver metastases, NLR is the only independent predictive marker.
Different cut-off values for NLR and PLR have been used in previous studies. To determine the cut-off value for NLR or PLR, most studies have used the usual cut-off (e.g. 3 or 5 for NLR) (12), some have considered the mean/median value of the NLR or PLR in their study population (13), and some have done ROC curve analysis (14). In the current study, a ROC curve was constructed and according to the maximum Youden index, a cut-off value of 2.66 and 182.58 was estimated to be the optimal cut-off value for NLR and PLR, respectively. Based on the calculated cut-off, 87% of the patients with high NLR and 86.7% of those with high PLR did not objectively respond to first-line chemotherapy.
High NLR and high PLR were both significantly associated with the low level of serum albumin in our study. This finding is consistent with previous studies that indicated albumin as an “inflammatory marker” rather than a “nutritional marker” among patients with CRC (15). Besides, hypoalbuminemia combined with C- reactive protein level, which is used in Glasgow Prognostic Score, has been shown to have a significant prognostic value in patients with cancer (16).
Most of the previous studies to assess the predictive significance of the above markers used NLR to predict pathologic complete response (pCR) after neoadjuvant chemotherapy (NAC) in various non-metastatic cancers. Overall, patients with NLR lower than the cut-off values were more likely to achieve pCR after NAC (17). Three studies have independently used NLR to predict response to chemotherapy in MCRC with contradictory results. In a retrospective study by Chua et al., unresectable MCRC patients with NLR > 5 were significantly less likely to respond to palliative chemotherapy compared to those with NLR < 5 (12). In contrast, Shibutani et al. did not find any relationship between NLR and response to chemotherapy in unresectable MCRC (14). In another study in 50 recurrent unresectable and advanced CRC patients, the better response was reported in those with NLR < 4, although the difference was not statistically significant (18). In the present study, to evaluate the relationship between NLR and PLR and chemosensitivity, we focused on the chemo naive patients; so, patients with metachronous metastases were excluded. In this regard, the patients in our study were more homogenous in comparison to the previous ones.
In our literature search, we found only one study, which has reported the association of both NLR and PLR with response to chemotherapy in MCRC. In a study by Wu et al. in 55 MCRC patients, those with NLR < 4 and PLR < 150 had better disease control after chemotherapy. There was also a trend for better objective response in patients with low NLR. They did not perform a logistic regression analysis to determine which variable is independently associated with response (19).
There are a limited number of studies, in which these two markers were compared in MCRC; all of them for predicting survival. Some of these studies have demonstrated that both NLR and PLR are significantly associated with lower survival, but after multivariate analysis, NLR remained the only independent factor (20-22). In a study by Zou et al., AUC for predicting survival was 0.748 for NLR and 0.690 for PLR and the authors concluded that NLR was the superior predictive factor in patients with CRC (23). In our study, AUC for predicting objective response rate for NLR (0.725) was greater than AUC for PLR (0.661) and NLR was the only independent predictive marker in multivariate analysis. On the other hand, there was a positive correlation between these markers in linear regression analysis. Taken together, these results might suggest that although PLR does have a predictive value, its association with response seems to be confounded by NLR.
The strongest evidence of the relationship between inflammation and cancer might be seen in CRC (24). There is evidence that inflammation affects the response to therapy, but the exact underlying molecular and cellular mechanism is not clearly understood. High NLR and PLR are both accompanied by relative lymphopenia. It has been proved that anti-tumor activity is related to lymphocytes dependent cell-mediated immunity (25). Lymphocytes usually CD3+ T cells, have an important role in the adaptive immune response in preventing the development and progression of CRC (26). High tumor-infiltrating lymphocytes (TIL) are also confirmed to be associated with more benefit from chemotherapy in different cancers most importantly CRC (27). High NLR is associated with relative neutrophilia. The role of Neutrophils in cancer progression and impaired response to therapy has been described. Neutrophils are recruited by tumors into the tumor microenvironment (TME), in which they transit to pro-tumorigenic type myeloid-derived suppressor cells (MDSCs). These cells not only suppress the presence of lymphocytes in the tumor site but also decrease the anti-tumor activity of TIL (28).
Unfortunately, post-treatment CBC was not available in half of the patients in this study; so, we were not able to assess the effect of response to systemic therapy on NLR alteration. Studies have demonstrated that normalization of NLR after chemotherapy is associated with better outcomes (12, 29). In a study by Nemoto et al. in patients with unresectable CRC, the overall survival and progression‑free survival were improved in patients with decreased post-treatment NLR compared to patients with increased posttreatment NLR (30). Whether using additional therapy to normalize pretreatment inflammatory markers would improve clinical response is not known. Today, there is no specific treatment to normalize the inflammatory network, but targeting mediators of neutrophilic origin, like monoclonal antibodies against IL-17A, has shown promising results to improve anti-VEGF (vascular endothelial growth factor) therapy in colon cancer (31). Investigating new approaches to “normalize the inflammatory network” to regain “a normal host response” might be more valued in the future (24).
There are some possible limitations in this study. First, we reviewed data of a small number of patients in a retrospective study; so, our result could not be generalized to all MCRC patients. Second, the comorbidities of the patients and side effects that might lead to dose modifications of the chemotherapy regimen were not taken into account.
5.1. Conclusions
NLR but not PLR in the pretreatment blood sample is an independent predictive marker of response to chemotherapy in CRC with synchronous liver metastases. Measurement of NLR as an inexpensive and readily available marker could be used to distinguish responders from non-responders and guide the treatment approach for MCRC.