1. Background
Gastric cancer is the fourth most common cancer globally and the second most common reason for cancer-related death after lung cancer (1, 2).
In terms of both mortality and prevalence, gastric cancer holds a special place among malignancies worldwide, especially in Asia. In Iranian men and women, gastric cancer is the first and third most common cancer, respectively (3).
Gastric cancer has a high propensity to involve lymph nodes and spread locally; the larger the tumor expansion is, the more invasive they are (4, 5).
The most effective predictor of overall postoperative survival (OS) is the metastasis of the lymph nodes. Hence, the status of the lymph node is considered the critical prognostic factor in gastric cancer (2, 6). Therefore, adequate treatment for all resectable GCs will be radical gastrectomy along with locoregional lymphadenectomy (2, 4).
According to the AJCC (UICC TNM classification) staging system of malignant tumors, at least 15 lymph nodes are required to be pathologically cleared for achieving a higher OS. According to the recent literature, extended lymphadenectomy can help avoid the locoregional invasion, while causing inevitable postoperative complications that impair the OS.
The lymph node ratio (LNR), which is defined as the ratio of metastatic lymph, nodes to the total number of resected lymph nodes and the number of positive lymph nodes can be considered the essential prognostic factors for early gastric cancer (EGC) (2, 4, 6, 7).
This study is conducted to evaluate the correlation of clinicopathological factors with overall survival and investigate the prognostic role of dissected lymph node count (DLNC), positive LNC (PLNC) LNR in patients with EGC, who underwent surgical resection with locoregional lymphadenectomy.
Despite much evidence in this regard, we believe the total number of dissected lymph nodes should be considered among prognostic factors, as it can negatively affect 1-year and 5-year survival.
2. Methods
The current retrospective cohort research is designed to assess the relationship between the DLNC, PLNC, and LNR and the 1-year and 5-year survival of patients who underwent either total or subtotal gastrectomy with locoregional lymphadenectomy. This study is conformed to the Declaration of Helsinki’s ethical guidelines, and all patients’ rights are respected.
All patients with resectable gastric masses referred to our center, one of the country’s main referral centers, underwent surgical resection of the tumor with curative-locoregional lymphadenectomy and lymph nodes were sent for further pathologic evaluations.
2.1. Data Collection
We only included patients with a confirmed diagnosis of resectable gastric adenocarcinoma made through an endoscopic evaluation. A total of 201 patients who underwent surgical resection associated with locoregional lymphadenectomy between 2010 and 2017 were retrospectively evaluated.
We obtained demographic characteristics of patients including age, gender and BMI, and clinicopathological data including their primary symptoms at the onset of diagnosis, T stage, grade (according to tissue differentiation), lymph node status, distant metastasis, lymphovascular invasion (LVI), number of regional lymph node removed and the result of pathologic examinations data on tumor invasion, lymph node status, and presence of distant metastasis, respectively. All patients were followed for at least 5 years, and their overall survival was determined as a time between the surgery and the date of the last follow-up or death.
TNM stages of the GC were determined according to the American Joint Committee on Cancer (AJCC) 7th edition staging system (8).
Patients were also contacted to ensure receiving chemotherapy or radiotherapy at any stage, recurrence, and vital status. Those patients who had missing lymph node data and incomplete records on LVA, grade, and removal of regional lymph nodes were excluded from the study.
2.2. Statistical Analysis
Statistical analysis was performed by using SPSS (IBM) version 16.0 software. The mean and median demographic and clinical characteristics of the sample population were mentioned. The two-tailed chi-square test was conducted to assess the importance of differences between the categorical variables. The independent sample t test was used to evaluate the correlation between LNR and clinicopathological characteristics. ANOVA test was used for assessing the relation between tumor grades, T stage, and lymph node status with the LNR. All reported P-values were two-sided, and also the statistically significant P-value was considered to be less than 0.05. Survival rates were calculated, using the Kaplan-Meier method, and the survival probability was estimated for overall cohorts and gender.
3. Results
A total of 201 eligible patients were included in this study. The mean age of our patients was 60.29 ± 13.32 years old, and the male to female ratio was 3:1. The median follow-up time was 36 months (range 24 to 84).
LVI was detected in 116 (57.7%) patients. During surgeries, 14.42 ± 8.52 lymph nodes were resected on average. The mean LNR in this population was 0.28 ± 0.32 with an average of 4.83 ± 6.61 positive lymph nodes found in each patient.
The association of DLNC, PLNC, and LNR with clinicopathological characteristics is summarized in Table 1 and 2, respectively.
| Characteristic | P-Value for Positive Lymph Node Count | P-Value for Resected Lymph Node Count | P-Value for Lymph Node Ratio |
|---|---|---|---|
| LVI | |||
| Positive | 0.07 | 0.017 | 0.232 |
| Negative | 0.181 | 0.949 | 0.216 |
| Differentiation of tumor | < 0.001 | 0.003 | < 0.001 |
| Depth of tumor (T) | < 0.001 | < 0.001 | < 0.001 |
| Lymph node status (N) | < 0.001 | < 0.001 | < 0.001 |
| Distant metastasis (M) | 0.018 | 0.009 | 0.035 |
| Time to metastasis | 0.0450 | 0.014 | 0.008 |
| Recurrence | 0.817 | 0.910 | 0.6 |
| Time to recurrence | 0.254 | 0.242 | 0.6 |
| Death | 0.001 | 0.008 | 0.003 |
Abbreviation: LVI, lymphovascular invasion.
aP-value <0.05 is considered statistically significant.
| Characteristic | No. (%) | P-Value |
|---|---|---|
| Age | - | 0.163 |
| Gender | - | 0.403 |
| BMI | - | 0.781 |
| Primary symptom | ||
| Dysphagia | 32 (15.9) | 0.301 |
| Abdominal pain | 49 (24.4) | |
| Anemia | 15 (7.5) | 0.171 |
| Hematemesis | 26 (12.9) | 0.462 |
| Weight loss | 52 (25.9) | |
| Vomiting | 23 (11.4) | 0.649 |
| No symptom | 36 (17.9) | 0.043 |
| Other symptoms | 14 (7) | 0.609 |
aP-value < 0.05 is considered statistically significant.
Clinical characteristics, including hematemesis, anemia, dysphagia, abdominal pain, weight loss, and vomiting were recorded on the first visit. After statistical analysis, our result did not reveal any association between LNR and primary symptoms (P-value > 0.05 for all).
The data show that a higher LNR is significantly associated with T stage, high grade, and death (with a P-value of < 0.001, < 0.001, and 0.003, respectively), but not with risk of recurrence or distant metastasis (with a P-value of 0.60 and 0.35, respectively). Our statistical analysis did not support any correlation between the onset of either recurrence or distant metastasis with LNR (P-value of 0.65 and 0.87 respectively). Similar associations were found for PLNC.
Table 3 demonstrates the role of the DLNC, PLNC, and the LNR in 1-year and 5-year survival within the study population. According to our results, a higher LNR is associated with higher 1-year survival. A higher PLNC is associated with higher 5-year survival, and a higher DLNC is associated with lower 5-year survival.
| Patients with Less Than 1-Year Survival | Patients with More Than 1-Year Survival | P-Value for Association with 1-Year Survival | Patients with Less Than 5-Year Survival | Patients with More Than 5-Year Survival | P-Value for Association with 5-Year Survival | |
|---|---|---|---|---|---|---|
| Mean DLNC | 15.60 | 15.18 | 0.731 | 20.40 | 14.72 | 0.002 (negative association) |
| Mean PLNC | 4.43 | 5.38 | 0.314 | 4.48 | 7.28 | 0.047 (positive association) |
| Mean LNR | 0.2336 | 0.3494 | 0.011 (positive association) | 0.2819 | 0.2845 | 0.970 |
Abbreviations: DLNC, dissected lymph node count; PLNC, positive lymph node count; LNR, lymph node ratio.
aP-value < 0.05 is considered statistically significant.
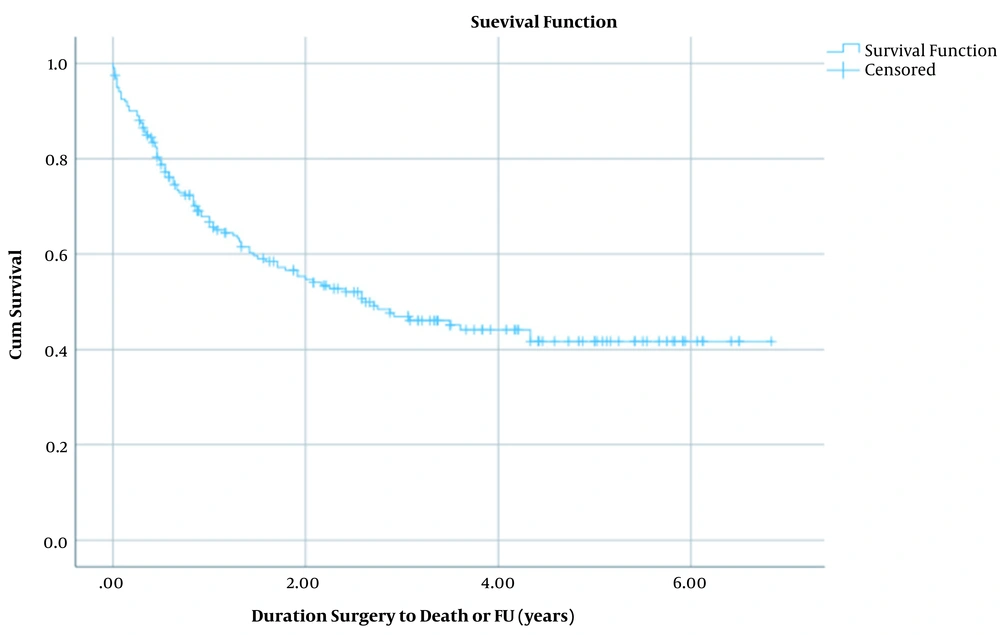
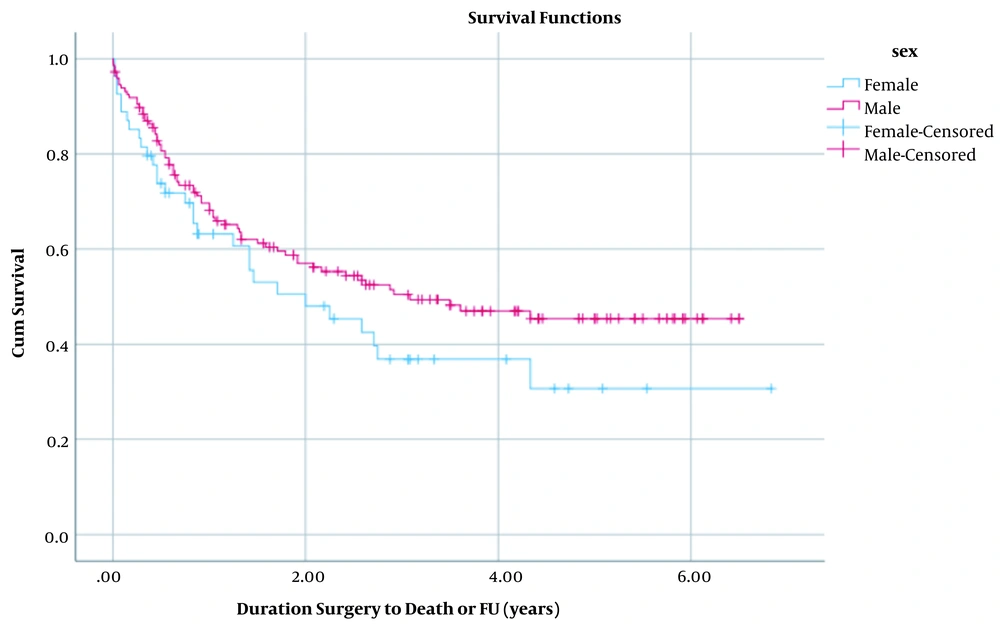
For assessing the overall survival, we used the surgery date as the start date of survival analysis (since the diagnosis date was missing in many of cases), and the endpoint for analysis was either the date of death (for the 100 deceased patients during the study period) or the follow-up date (for the 101 survived cases) that happened for all the cases in February and March 2020. This period was defined as the surviving duration; the analysis’s failure event variable was patients’ death. Censored cases (right-censored mainly due to surviving more than the endpoint of the study) were also included in the analysis. Survival analysis was conducted by Kaplan-Meier method curves for all patients and stratified by sex. Differences between analyses of two sexes were measured by the log-rank test. Also, life table analysis was done for 12-months intervals (Figures 1 and 2).
The association of LNR, DLNC, and PLNC with outcomes in patients, who received any type of comprehensive cancer treatment programs (neoadjuvant and adjuvant chemotherapy and preoperative or postoperative radiotherapy), are separately assessed via an independent sample test. Unfortunately, 55 patients did not respond to our call and their medical history was missing (Table 4).
| Type of Comprehensive Cancer Treatment Program | Total (No.) | 2-Year Survival, No. (%) | Correlation of LNR with Survival (P-Value) | Correlation of Positive Lymph Node Count with Survival (P-Value) | Correlation of Resected Lymph Node Count with Survival (P-Value) |
|---|---|---|---|---|---|
| Neoadjuvant chemotherapy | |||||
| Yes | 62 | 18 (29) | 0.173 | 0.114 | 0.114 |
| No | 84 | 30 (36) | 0.006 | 0.017 | 0.020 |
| Adjuvant chemotherapy | |||||
| Yes | 74 | 29 (39) | 0.343 | 0.128 | 0.056 |
| No | 72 | 19 (26) | < 0.001 | 0.002 | 0.008 |
| Preoperative radiotherapy | |||||
| Yes | 27 | 7 (26) | 0.912 | 0.364 | 0.224 |
| No | 119 | 41 (34) | 0.001 | 0.001 | 0.008 |
| Postoperative radiotherapy | |||||
| Yes | 54 | 16 (30) | 0.999 | 0.734 | 0.84 |
| No | 92 | 32 (35) | < 0.001 | <0.001 | <0.001 |
Abbreviation: LNR, lymph node ratio.
aP-value < 0.05 is considered statistically significant.
4. Discussion
Despite the recent development in GC screening and staging techniques, such as CT scan and endoscopic ultrasonography, the possibility of clinical under-staging is still substantial (4).
The extent of lymphadenectomy in EGC strongly depends on lymph node status and the spread of the tumor. A lower probability of lymph node metastasis is expected for EGC as compared to the advanced forms. Several factors, including the macroscopic and microscopic appearance and size of the tumor, submucosal invasion, tumor grading, and lymphovascular invasion have been significantly associated with a higher risk of lymph node metastases EGC (9).
The Japanese Gastric Cancer Association (JGCA) has determined a criterion for endoscopic indications of lymph node dissection. Also, they advised a D1 or D1 plus lymphadenectomy procedure in early forms not suitable for endoscopic treatment and have clinically negative nodes. JGCA guidelines recommend a D2 dissection only when lymph nodes are clinically positive (4).
A recent study from Seoul National University Hospital (SNUH) on the appropriate extent of lymph node dissection authors similarly concluded that the D1 plus technique for mucosal cancer and an expanded dissection to the D2 level for submucosal cancer should be considered to ensure complete removal of metastatic LNs (10).
Despite much evidence for proving the introduced approach by JGCA, the clinical setting is different in the West. Endoscopic resection, which is both a treatment approach and a staging procedure, is less frequently utilized in the West. As a result, the diagnosis of EGC is based mainly on the clinical findings in most cases. Consequently, there is a considerable risk of clinical understaging, which is indeed associated with a missed diagnosis until the occurrence of advanced nodal involvement. Accordingly, the GIRCG guidelines advise a D2 lymphadenectomy even in clinically early forms that are not suitable for endoscopic treatment. (11) Amid the agreement on the complications and postoperative mortality following a D2 lymph node dissection, the GIRCG group only accepts a more limited procedure (D1 plus) for selected cases (high-risk patients, early forms with favorable pathological characteristics) (12, 13).
Although the extended lymphadenectomy can securely avoid metastasis and further regional involvement, it has long-term results and can lead to poor outcomes in some cases. Although many studies have reported a positive correlation between survival and DLNC in gastric or colon cancer, some others have found no evidence supporting this hypothesis. Several arguments have been raised against the role of stage migration in explaining the correlation between the DLNC and survival as an increase in DLNC did not necessarily correlate with a change in node positivity (14-16).
This study aims at helping surgeons in choosing the optimal treatment approach. Surgical treatment with adequate lymphadenectomy could offer a high probability of cure. Because lymph node status is the strongest prognostic factor for EGC, this study aims at predicting the 5-year and 10-year cancer-related survival according to the DLNC, PLNC, and the LNR in patients with EGC, who underwent gastrectomy and lymphadenectomy.
In our study population, 5-year survival was about 40% considerable to 12% in similar studies. This considerable difference can be due to the greater awareness of people for early referral and routine screening, gastroenterologists’ cooperation, the development of our diagnostic and surgical equipment, and the surgical technique that was consistently associated with locoregional lymphadenopathy while avoiding extreme lymph node dissection.
According to our data analysis, a higher LNR is associated with higher 1-year survival, a higher PLNC is associated with higher 5-year survival, and a higher DLNC is associated with lower 5-year survival. In other words, LNR is a better prognostic factor for 1-year survival, while for 5-year survival, we need to consider the DLNC. These findings emphasize the need for an optimal approach to limit the number of total dissected nodes and achieve a higher LNR. The goal is to dissect as a less negative node as possible to avoid post-operative complications of extended lymphadenectomy.
According to the latest population studies (17), the incidence and mortality rates are higher in men compared with women. In contrast, we observed a lower survival rate among females (Figure 2). However, the difference between the survival rate between males and females was not significant (P-value = 0.146) and can stem from the lower number of women diagnosed with GC and be included in our study (we had a male to female ratio of 3:1).
We also evaluated the correlation between demographic and clinicopathological characteristics and DLNC, PLNC, and LNR and reappraisal of these measures as predictors of survival in GC when done through locoregional lymphadenectomy.
Our results illustrate that DLNC, PLNC, and LNR are not correlated with demographic data such as age, gender, and BMI with a P-value > 0.05 for all.
Among primary symptoms, we did not find any correlation with any of the symptoms including dysphagia, abdominal pain, anemia, hematemesis, weight loss, or vomiting, and the LNR. However, patients who were asymptomatic and their diagnoses were made at the routing screening program had significantly lower LNR (P-value = 0.04).
Interestingly, our data did not support any correlation between DLNC, PLNC, and LNR and LVI in this group of patients, who all had early stages of GC.
In general, it is believed that the prognosis is linked to clinicopathological conditions (such as the location, invasion depth, distant metastasis, nodal status, and LVI) and treatment approach (such as surgery and dissection of the lymph nodes). Clinical and lymph node staging will help the clinician better determine the progression of the tumor, establish a detailed individualized treatment decision, and analyze diagnosis and prognosis (2).
Accordingly, we assessed the relationship between the DLNC, PLNC, and LNR and pathological features. We achieved a significant relationship between higher T stage and lymph node status (P-value < 0.05 for all, presented in Table 1) and DLNC, PLNC, and LNR.
Bilici et al. conducted similar research to determine the prognostic significance of metastatic LNR and compared it to the number of lymph node metastasis in pN3 gastric cancer. By retrospective analysis of 207 patients, they reported a metastatic LNR of 0.75 to be the best cut-off value to determine the prognosis of patients with pN3 gastric cancer (P = 0.001). In contrast to our findings, they demonstrated that Lymph node and peritoneal recurrences’ risk were significantly higher in patients with LNR > 0.75 (P < 0.05) (18). However, our result does not support this association, and higher LNR was not correlated to a higher risk of recurrence or distant metastasis within our population (P-value of 0.6 and 0.35 respectively).
We found that distant metastasis occurred significantly earlier in patients with higher LNR. But, the time to recurrence was not statistically associated with LNR. Our data supported that LNR can be considered a predictor of survival; we found significantly higher LNR in patients who did not survive until the end of the follow-up period (P-value of 0.003).
Wang et al. compared the LNR-based staging system’s bias with the TNM-based staging for gastric cancer. They analyzed 18 043 patients from the database and reported a significantly lower bias for the LNR-based system (12% vs 57%) (19).
Lee et al. identified that higher DLNC correlates with better survival in patients with pN2, pN3a, and pN3b gastric cancer through a multicenter cohort study. Also, they emphasized that LNR appears to be a better predictor for survival than the N category because the LNR formula includes the DLNC (14).
Similarly, Zhu et al. published a review of 27 studies about the association between LNR and OS. They concluded that a higher LNR is significantly related to shorter overall survival in patients with gastric cancer, even when subgroup analysis was performed, using all the different factors. They also illustrated that LNR can be an independent prognostic indicator in patients with gastric cancer and should be considered a parameter in future staging systems (2). However, they did not provide conclusive information about the DLNC itself.
Based on our findings, the best approach for lymph node dissection would be the dissection of the least negative nodes and the most positive nodes. Our findings proved that higher LNR and lower DLNC are associated with higher 5-year survival. These findings emphasize the crucial role of intraoperative evaluation of lymph nodes, thus avoiding negative lymph node dissection.
Another controversy is about the prognostic significance of LNR when preoperative chemotherapy is applied to patients with advanced GC. In the east, the most effective combined therapy after resection of advanced GC is adjuvant chemotherapy, while western and European countries, usually apply neoadjuvant chemotherapy (20-25).
It is expected that if neoadjuvant chemotherapy is done effectively, it would modify the lymph node status through downstage (26). Currently, in Iran, neoadjuvant chemotherapy is routinely performed for patients before surgical management of proximal gastric carcinoma and tumors of the cardia.
To address this controversial thought, we performed a multi-variant analysis to assess the association between LNR and survival in patients who received different types of comprehensive cancer treatment programs. Interestingly, the Prognostic significance of DLNC, PLNC, and LNR by type of received comprehensive cancer treatment program was also assessed. Interestingly, our data reveal that lower DLNC, higher PLNC, and higher LNR are statistically associated with higher survival only in patients who have not been received chemotherapy or radiotherapy before or after the surgeries. Still, a similar association was not found in patients who received comprehensive cancer treatment (Table 3).
According to this result, we incline that all types of combined therapies can modify lymph node status. Thus, we emphasize that DLNC, PLNC, and LNR can be considered predictors of overall survival only in early-stage GC cases, who had not received courses of chemotherapy or radiotherapy.
Hung et al. have recently presented an accurate LNR-based prognostic model for predicting the survival outcome after D2 lymphadenectomy in GC patients with metastasis to more than 15 regional lymph nodes. Their model was developed by measurement of 5 separate factors, including the T-classification, LNR, carcinoembryonic antigen level, Eastern Cooperative Oncology Group performance scale, and adjuvant chemotherapy. This model has established the T stage, CEA, and ECOG PS as prognostic factors for patients with advanced stages of GC, who have undergone curative surgeries (27).
Our study has some limitations. Firstly, we did not stratify the outcome and overall survival; so, calculating the cut-off for the LNR associated with poor prognosis was not applicable. Secondly, we did not categorize our patients into early and advanced staged tumors. Finally, we did not compare the significance of LNR-based staging with the TNM-staging system in predicting the OS. Future studies with a larger population are recommended to compare these two staging systems.
4.1. Conclusions
According to this study, LNR, DLNC, and PLNC are significant prognostic factors for EGC. Choosing the optimal approach, through which fewer negative lymph nodes are dissected, is crucial in increasing overall survival and extended lymphadenectomy cannot necessarily benefit patients with EGC. Also, our finding limits the prognostic significance of LNR, DLNC, and PLNC to patients who did not receive comprehensive cancer treatment programs.