1. Introduction
Primary breast lymphoma (PBL) is a rare extranodal lymphoma (1). It is defined as the presence of lymphoma in the breast without the involvement of other extramammary regions (2). It is mainly prevalent among women while being rare among men (3). Considering its higher prevalence in women, hormonal factors are considered as probable causes (2).
The average age of patients is 66-67 years. Most patients are diagnosed at stages 1 and 2 (4). The most common pathology is diffuse large B cell lymphoma, though other pathologies such as marginal zone lymphoma, follicular lymphoma, Burkitt lymphoma, anaplastic large cell lymphoma, peripheral T cell lymphoma, small lymphocytic lymphoma, lymphoplasmacytic lymphoma, mantle cell lymphoma, and Hodgkin lymphoma have also been reported (2). The most common symptom at the time of diagnosis is the presence of a painless mass. Other symptoms include pain, inflammation, lymph node palpation, and occasionally as an accidental findings in imaging (5). The therapeutic options available so far include surgery, radiotherapy, and chemotherapy (3). In this study, we reported a patient with primary diffuse large B-cell lymphoma (DLBCL) breast lymphoma who experienced an isolated relapse in the contralateral breast after treatment.
2. Case Presentation
The patient was a 54-year-old woman with no family history, presenting with a mass in her right breast as primary symptom. There were no B symptoms. A hypoactive nodule with vascularity plus lymphadenopathy in the ipsilateral axilla was found in sonography.
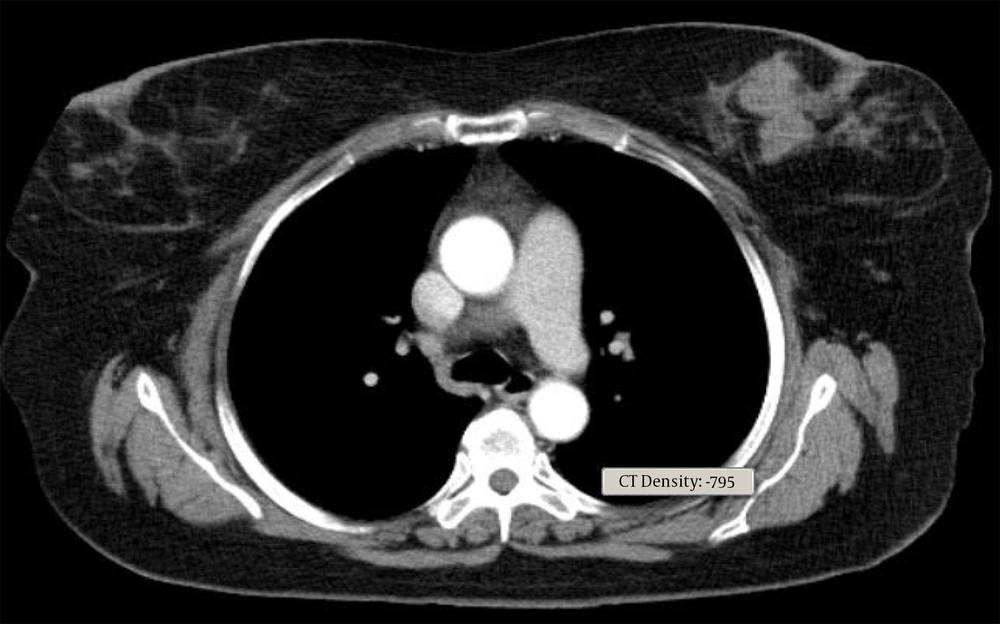
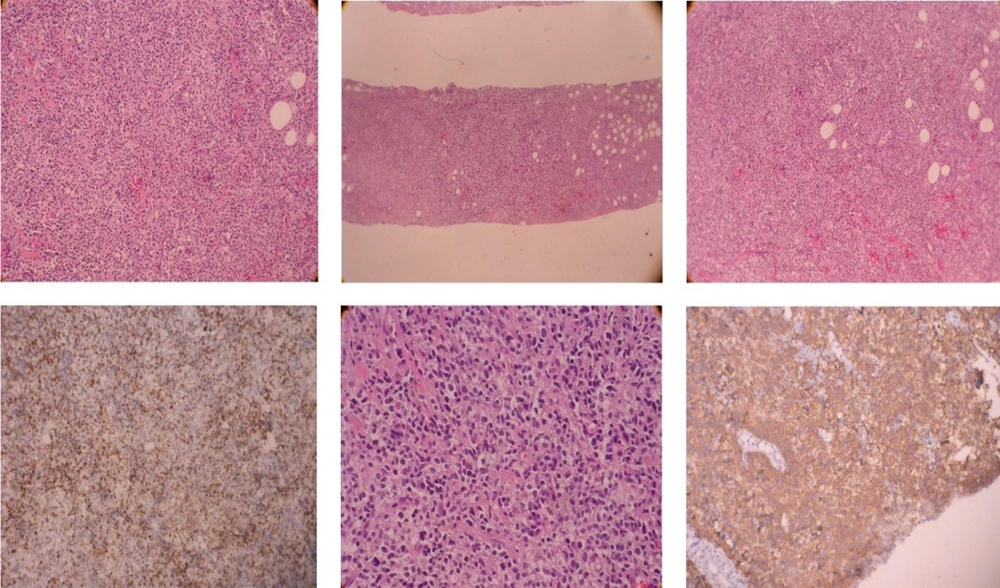
Core needle biopsy of the breast mass was done and DLBCL was reported. Cervical, thoracic, and abdominopelvic CT scan as well as BMB, did not show involvement of other sites. The patient underwent 6 courses of chemotherapy with an R-CHOP regimen followed by radiotherapy of the breast and regional lymph nodes with a dose of 40 Gy. In PET scan done three months later, the patient was in complete remission. Around one year later, the patient noted a mass in the contralateral breast (Figure 1). In the core biopsy, the pathology was misdiagnosed as mantle cell lymphoma at first but after review of morphologic study and IHC staining DLBCL of non-germinal center was confirmed. CD19, CD20, BCL2, MUM1, SOX11 markers were positive in IHC staining and CD10, Cyclind1, BCL6 were negative (Figure 2). In the PET scan performed, hypermetabolic multifocal soft tissue density mass in the left breast with SUV max 14 was reported, while there was no uptake in other regions. The patient received three courses of chemotherapy with R-CHOP regimen and the treatment was tolerated well. There was no sign of disease in the post chemotherapy CT scan. Radiation therapy including whole breast and regional lymph nodes with a dose of 40 Gy was done. There were no serious radiation side effects. In 3-month follow-up, the patient was disease-free.
3. Discussion
PBL is a rare form of extranodal lymphoma so there are a limited number of relevant studies (1). In this disease, there are 2 clinicopathologic scenarios: (i) the unilateral type similar to breast carcinoma being more common, that can occur at any age; (ii) bilateral and diffuse-type in pregnant and breastfeeding women, and has a pathology similar to Burkitt lymphoma (6). Most pathological cases are high-grade and DLBCL (3). In some studies, ALCL (anaplastic large-cell lymphoma) in women with breast implant has been reported, which is mostly localized (7). In imaging studies, such as CT scan, mammography or sonography, PBL is usually oval or round with circumscribed margin, thus fibroadenomas, cyst and breast carcinoma could be considered as radiological differential diagnosis. Other clinical differential diagnosis include sarcoma, mammary dysplasia and inflammatory carcinoma. Other imaging modalities such as PET scan is not specific and could mimic carcinoma (8).
Definitive diagnosis is based on pathologic features. Epithelial markers such as cytokeratin are positive in ductal carcinoma and negative in lymphoma. Differentiation of lymphoma subtypes is possible by IHC and molecular features such as translocations. Ki67 proliferation rate of more than 40% demonstrates high-grade lymphoma and below 10% to 20% illustrates low-grade lymphoma (9).
In the pattern of relapse of patients with primary breast lymphoma, there is a tendency for extranodal relapse (2). So, we try to exploring the pattern of relapse and the treatments available for PBL.
In the study by Aviles et al., covering 96 PBL patients, combination treatments i.e. radiation and chemotherapy with CHOP regimen, lead to the best outcome compared to a single modality, with the most relapses occurring in CNS. Thus, CNS prophylaxis might be considered in these patients (10).
In the study by Jeanneret-Sozzi et al., investigating 84 PBL patients, pattern of relapse was mostly lymphatic, followed by CNS and BM. Other sites of relapse were skin, lungs, spleen, muscles, orbit, stomach, Waldayer ring, and liver. The local relapse rate in that study was 12% (3).
In the study by Ryan et al., 204 patients with PBL were investigated including 5% of cases being bilateral. The patients received single modality or combination treatment including 80% chemotherapy with or without surgery or chemotherapy with radiation in 62% of patients who received systemic therapy. The median overall survival (OS) in these patients was 8 years. The relapse occurred in the ipsilateral breast during 2.6 years of the primary treatment, while relapse in the contralateral breast occurred in 13.3 years. In the relapse of unilateral cases, 4% was ipsilateral and 9% was contralateral. Breast radiotherapy lowered ipsilateral relapse rates. With radiation to the regional nodes, regional relapses tended to diminish (11).
The multicenter study of Yhim et al. assessed 33 primary breast DLBCL. Treatment schedule consisted of 6 cycles of R-CHOP and 4 doses of IT-MTX. Local radiation was not administrated. After the median follow-up of 46.1 months, 2 years progression free survival was 81.3%. Four patients relapsed in the CNS and 2 patients in the breasts. This study showed chemotherapy with rituximab and CNS prophylaxis without routine local radiation may be a sufficient approach (12).
We reviewed some studies including primary breast DLBCL in regarding primary treatment modalities and the site of relapses in the Table 1.
| Study/Year | Number of Patients/ Pathology | Primary Treatment Modalities | Sites of Relapse |
|---|---|---|---|
| Ryan et al., 2008 (11) | 204, DLBCL | Chemotherapy, +/- surgery, +/- RT, 8 patients CNS prophylaxis | 32 breast, 13 regional node, 11 CNS, 11 other nodal site, 28 other extranodal, 2 BM |
| Yhim et al., 2012 (13) | 25, DLBCL | 3 - 4 cycles R-CHOP + RT, Or 6 - 8 cycles R-CHOP +/- RT, 2 patients CNS prophylaxis | 2 isolated nodal, 5 breast or CNS |
| Hosein et al., 2014 (14) | 76, DLBCL | CHOP +/- Rituximab, Or Hyper-CVAD +/- Rituximab, +/- RT, Or no chemotherapy, 9 patients CNS prophylaxis | 10 breast: 5 ipsilateral, 3 contralateral, 2 bilateral; 12 CNS |
| Ou et al., 2014 (15) | 23, 21;, DLBCL | 3 mastectomy, 1 RT, 7 Rituximab, All chemotherapy | 2 contralateral breast, 3 CNS, 4 distant nodes |
| Zhang et al., 2016 (16) | 24, DLBCL | 1 Surgery, 4 - 6cycles CHOP or chop like, +/- Rituximab (10), +/-RT (12), 4 CNS prophylaxis | 1 BM, 1 breast, 1 skin |
| Yhim et al., 2020 (12) | 33, DLBCL | 6 cycles R-CHOP, + CNS | 4 CNS, 2 breast: 1 ipsilateral, 1 contralateral |
Abbreviations: RT, radiation therapy; CNS, central nervous system; CHOP, cyclophosphamide, adriamycin, vincristine, prednisolone; Hyper-CVAD, cyclophophamide, vincristine, adriamycine, dexamethasone, cytarabine, methotrexate, leucovorin.
The early diagnosis of primary breast DLBCL, and differentiating it from other benign and malignant breast tumors, including other types of Hodgkin and non-Hodgkin lymphoma have an important role in treatment and prognosis, and patients would be spared from unnecessary procedures such as surgery. As in our case, there is a rare but possible incidence of metachronous PBL, thus close follow-up by physical examination and imaging may detect disease in the early and curable stage.
3.1. Conclusions
In PBL, relapse in the contralateral breast is probable and the patient should be under regular follow-up and visit after the primary treatment for early diagnosis of the relapse.