1. Background
Patients eligible for immune checkpoint inhibitor (ICI) treatment increased from 1.5% in 2011 to 43.6% in 2018 (1). Therefore, the number of patients presenting at Emergency Departments (ED) with immune mediated toxicities has proportionally grown as well (2). Immune mediated side effects vary from those of traditional chemotherapy and require different management (3). Early recognition and timely steroid initiation are important (4).
2. Objectives
The objective of this multi-institutional audit was to assess the knowledge of ED physicians on indications of ICI treatment and management of its related toxicities.
3. Methods
In the context of a clinical audit, a multiple-choice questionnaire (see Supplementary File) was developed to assess the knowledge of ED physicians on ICI indications, recognition, and management of immune-mediated toxicities. After approval by the Immune-oncology group at Bart’s Cancer Centre, the questionnaire was delivered to ED physicians across the 6 largest EDs in London: (1) The Royal London, (2) The Royal Free, (3) Guys and St Thomas, (4) St George’s, (5) Queen’s Romford, and (6) Whipps Cross Hospitals. Participating clinicians included all levels of trainees and ED physicians. Junior grades were defined as all non-attending/consultant grade practitioners. Paper questionnaires were distributed during weekly ED educational sessions, followed by a short training on the management of immune-mediated toxicities. Participation was voluntary with permission obtained previously from department leads. All responses were anonymized, with only the grade and hospital of the ED practitioner known.
The questionnaire included 7, single- or multiple answer, multiple choice questions. A correct answer required all boxes ticked correctly. Likert items in ordered response levels were used to assess a participant’s confidence in their answers (‘completely unsure’, ‘somewhat unsure’, ‘somewhat confident’, and ‘confident’) (5).
Questions were designed to evaluate the participants’ ability: (1) to recognize ICI drugs amongst other anti-cancer agents and their use in the first- line setting in the commonest cancer indications (non-small-cell lung carcinoma, breast cancer, renal cell carcinoma, and melanoma); (2) to identify immune-mediated toxicities (rash, diarrhea, transaminitis) and their management (antibiotics, corticosteroids); (3) to recognize pharmaceutical interactions with ICIs; (4) to estimate the treating physician’s confidence in their responses; (5) to estimate the treating physician’s confidence in the information provided by the patient on their current treatment regimen. This question was non-clinical hence not scored.
A single site re-audit was performed, 10 months following an embedded intervention, to assess longer term learning outcomes. This intervention consisted of a one-hour interactive ICI seminar delivered by the Bart’s clinical trials team to ED staff. Learning points incorporated ICI prevalence, indications, toxicities, and their management – in addition to discriminating features from other forms of treatments, and directions to local guidelines and expertise.
Statistical analysis was performed to compare and calculate average questionnaire scores. Responses given by junior grade doctors and consultants were compared with Student t-test. All analysis was performed using a statistical software package (ToolPak).
4. Results
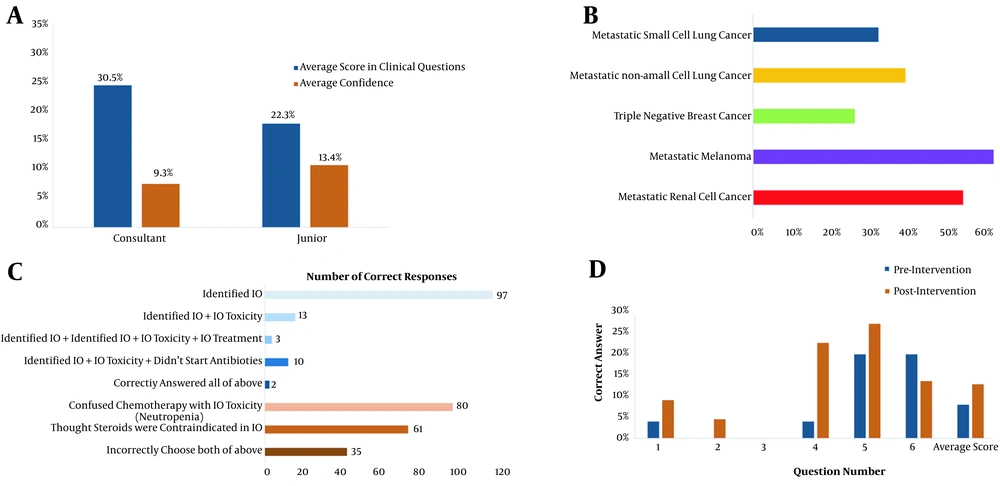
Between March 2019 and September 2019, the multiple-choice questionnaire was delivered in 6 EDs in London. Amongst 126 participants, 80% (101/126) were junior grade and 20% (25/126) were specialist ED consultants. There was no significant association between clinician's seniority and overall score reached on the questionnaire or confidence in responses (Figure 1A). The junior grade clinicians answered 22% of questions correctly compared to 31% by ED specialist consultants (P = 0.97). Furthermore, the confidence rate in the correct response was equally low in both groups (13.4% of junior grade clinicians vs. 9.3% of ED specialist consultants, P = 0.40).
56% (71/126), 49% (62/126), and 36% (45/126) of participants identified correctly ICIs as the first-line treatment regimen for melanoma, renal cell carcinoma, and non-small cell lung cancer, respectively (Figure 1B). This demonstrates that tumor types where ICI has been in use longer, are more recognized by ED physicians.
A, comparison of questionnaire scores vs confidence in responses. There was no statistically significant difference between scores of ED Juniors and attending physicians/ consultants (P = 0.97) or between reported confidence between scores of ED juniors and attending physicians/consultants (P = 0.40) (*junior grades were defined as all non-attending/consultant grade practitioners. Question 7 was non-clinical hence not scored); B, correct identification of an immunotherapy agent (IO) as a 1st line cancer treatment for common tumour types; C, correct management of a Grade 2 ICI-mediated colitis. Candidates were required to identify Pembrolizumab as an IO, recognize diarrhoea as an immune-mediated toxicity, that the correct management was oral steroids and that febrile ICI patients should not receive antibiotics empirically; D, re-audit of Royal London Hospital 9 months following initial audit and educational intervention with scores labelled by individual questions. An average score of 8% (n = 25) pre-intervention and 13% (n = 25) post-intervention (P = 0.31). Both cohorts included 4 consultants and 21 junior emergency physicians each.
Overall, 90% (113/126) of the participants identified correctly cisplatin as a chemotherapy agent and 77% (97/126) recognized pembrolizumab as an ICI agent (Figure 1C). However, 96% (121/126) also identified other agents (bevacizumab, imatinib) incorrectly as ICIs. The average score for responders who were “very confident” was 0 compared with 2 for responders who were “completely unsure”. This suggests that responders who were more confident were also more likely to score incorrectly.
The majority of ED physicians answered correctly that diarrhea >10 episodes/day (67% (85/126)) and skin rash involving ≥ 20% of body surface area [71% (89/126)] are established side effects of ICIs, requiring emergency treatment. However, less than half of the participants [47% (59/126)] identified transaminitis as an ICI-related side-effect. There was no association between confidence and accuracy of responses (P = 0.40).
When asked about the optimal management of diarrhea caused by ICIs, only 29% (37/126) chose the correct treatment option. Most ED physicians [76% (92/126)] answered correctly that patients receiving chemotherapy and presenting with fever at ED should receive empirical antibiotics. However, almost half of the participants [49% (62/126)] would also have treated patients on pembrolizumab with empirical antibiotics.
Among the participants, 94% (119/126) incorrectly selected drugs with potential ICIs interactions and in particular, 52% (65/126) thought corticosteroids would have significant drug-drug interactions with ICI.
When asked whether clinicians thought that patients knew the type of anti-cancer drug they were receiving, 40% (50/126) of participants thought that only half of their patients were actually aware of their exact treatment regimen.
Ten months following the initial audit and educational intervention, a re-audit at Royal London Hospital was performed (n = 25 pre-intervention, n = 25 post-intervention). The total average correct score of the questionnaire pre- and post-intervention was 8 and 13%, respectively (P = 0.31), suggesting a lack of durable long-term impact of the educational intervention (Figure 1D).
5. Discussion
As treatment options with ICIs in the commonest cancer types increase, patients presenting to EDs with immune-mediated toxicities will likely rise as well. Our results showed a good understanding and managing related side-effects of chemotherapy across all junior and senior ED medical staff. However, ED physicians scored overall less well on ICI-related questions.
Junior grade doctors and consultants scored similarly overall, with no direct association between confidence rating and score. One of the underlying causes for this might be the shorter time of ICI use in clinical practice compared to chemotherapy.
The questionnaire was designed to assess a basic understanding of ICI usage and toxicities relating to the acute management of ICI patients. Not all ED physicians correctly selected malignancies where ICIs are currently first-line UK treatment options suggesting that the prevalence of patients with immune-mediated toxicities presenting to ED is underestimated.
Furthermore, while ED physicians could recognize and manage related side-effects of chemotherapy quickly, ICI treatment toxicities were more likely to be missed. This is important as early recognition and appropriate treatment increases the likelihood of resolving immune-mediated side-effects and resuming anti-cancer treatment (6).
Our audit showed that, despite educational intervention, there was no statistically significant change in the questionnaire outcome (8% pre-intervention vs 13% post-intervention) at a 10-month interval. This demonstrates the need for structured education in ED departments and earlier education in undergraduate training.
Our study had several shortcomings such as the unbalanced number of participants between junior grade doctors and consultants. However, our ratio of junior and senior clinicians (4: 1) reflects the real-world structure of an ED department in the UK. Frequent clinician rotations in ED departments constitute a further limitation which may account for the ineffectiveness of the educational intervention. A re-audit was performed at a single ED, however, the number of post-intervention responses obtained were equal to the number of responses pre-intervention with a balanced number of consultants and juniors in the both groups (4 consultants and 21 junior ED physicians each). Additionally, questions relating to the ability of retrieving appropriate acute oncology clinical guidelines to inform patient management (e.g. trust/national guidelines) might have been useful to include in the questionnaire. However, such guidelines should not necessarily negate the need for a basic understanding and recognizing immune therapy related toxicities.
Further studies need to be done in order to assess understanding immune therapy nationally, to increase ICI awareness amongst ED clinicians and to incorporate ICI education into undergraduate medical training. By raising this important issue early, we hope effective measures can be taken to improve patient management pathways for the rapidly increasing number of oncology patients on ICI treatments.
5.1. Conclusions
Knowledge and management of immune mediated toxicities is inferior compared to chemotherapy across physicians working in major ED departments in London. This survey highlights the need for increased education on ICI amongst ED clinicians.