1. Background
Central nervous system (CNS) neoplasms comprise a group of different tumor, which are anatomically close to each other, but differ in terms of morphology, site, and clinical behavior (1). CNS tumors statistics are estimated by grouping all malignancies, originating from all CNS anatomic sites including brain, meninges, spinal cord, cranial nerves, and other CNS localization with degrees of malignancy, ranging from benign to aggressive (2). Improved diagnostic imaging, radio isotope imaging, and computed tomography have been resulted to high level of detection rate and better differential diagnosis of CNS tumors. Moreover, the specificity of tumor diagnosis and manifest increase in specific tumor types is due to improvement in histopathological techniques (3).
Reports in the literature indicate that world-wide variations exist in the pattern of CNS tumors with respect to incidence rate anatomical locations, gender preferences, and frequency of histological types. To a large extent, treatment modalities and outcome are influenced by histological subtypes and location. Hence, data on both parameters are useful for future research in any given locality.
The aim of this study is presenting the estimation of brain and CNS tumors frequency and also depicts tumor distribution according to age, sex, histological type, laterality, and anatomical location. It covered a 7-year period (2006 - 2013) that was based on the records of department of pathology, Razavi Hospital, Mashhad, Iran. This is a hospital-based, rather than population-based study; the results is expected to reflect more or less fair image of epidemiology of CNS tumors in north-east of Iran. However, it serves as a basis for future studies.
2. Methods
2.1. Data Collection
The data were directly collected from patients’ surgical pathology and diagnostic reports, Razavi Hospital, Mashhad, Iran. Data included cases of malignant and non-malignant primary brain and CNS tumors. Data were provided on 1,164 primary brain and CNS tumors diagnosed during 2006 to 2013. Due to the duplicate records, and invalid site or histology, 36 records were omitted from a data file analyses, because their information were not recorded. Patient’s characteristics, anatomical location, tumor type, histology’s and tumor grade were analyzed. Data were requested for tumors during 2006 to 2013 at any of the following sites: ICD-O-3 topography codes: meninges (C70.0), Cerebral meninges (C70.0), brain (C71.1 - 71.4), Ventricle (C71.5), Cerebellum (C71.6), brain stem (C71.7), other brain (C71.8, C71.9), spinal cord and caudaequina (C72.0), Cranial nerves (C72.2 - C72.5), pituitary and pineal glands (C75.1 - C75.3), and cranial nerves (72.3) (4).
2.2. Measurements and Analysis
The histology groupings used in this report are broadly based on the World Health Organization (WHO) categories for CNS tumors (1). Gliomas are the tumors that originate from glial cells, which are including astrocytoma, glioblastoma, ependymoma, mixed glioma, oligodendroglioma, NOS, and a few more histologies. In this study, astrocytoma was characterized by the ICD-O-3 histology codes, 9,401, 9,421, 9,424, 9,400, including Anaplastic astrocytoma, Gemistocytic astrocytoma, Oligoastrocytoma, Pilocytic astrocytoma, Pleomorphic xantoastrocytoma, Pilomyxoid astrocytoma, Diffuse astrocytoma, Mixedoligoastrocytoma, and Low-grade astrocytoma. Diffused fibrillary astrocytoma and fibrallary astrocytoma were categorized as fibrillary astrocytoma by histology code 9,420 (4).
Counts, proportion, ratio, and other statistics were calculated, using SPSS statistical software. The frequency of each gender and male female ratio was calculated for CNS in general and histological subtype tumors. And distribution for histology grouping by children (0 - 12 years), adolescent (13 - 19 years), and adult (≥ 20 years) was estimated. Moreover, the data were analyzed based on 10-year age grouping to calculate age-specific frequency. On the other hand, a time frame was considered in this study to detect changes in the distribution pattern.
This study was approved in ethical committee and research department of Razavi Hospital, Mashhad, Iran.
3. Results
Between 2006 and 2013, a total of 1,164 cases, consisting of 577 (49.6%) males and 587 (50.4%) females had histological confirmed diagnosis of CNS tumors in Razavi Hospital. Their age varied from 2 to 92 years. Among them, 49 (4.2%) were children (2 - 12 years), 29 (2.5%) adolescents (13 - 19 years), and 1,086 (93.3%) adults (≥ 20 years). Totally, 1,105 primary and 59 metastasis tumors, including 1,057 brain tumors were analyzed. According to 2003 WHO grading system for CNS tumors, primary tumors consisted of 541 cases for grade I, 40 cases for grade II, 203 cases for grade III, and 187 cases for grade IV. The incidence of CNS tumors increases with age from approximately 30 years old onwards, that is in common with all other adult cancers. The frequencies for both brain and spinal tumors reached the highest at 51 to 60 years old. The age distribution curve exhibits a decrease in number in those over 70 years, which is explained by co-morbid conditions like strokes.
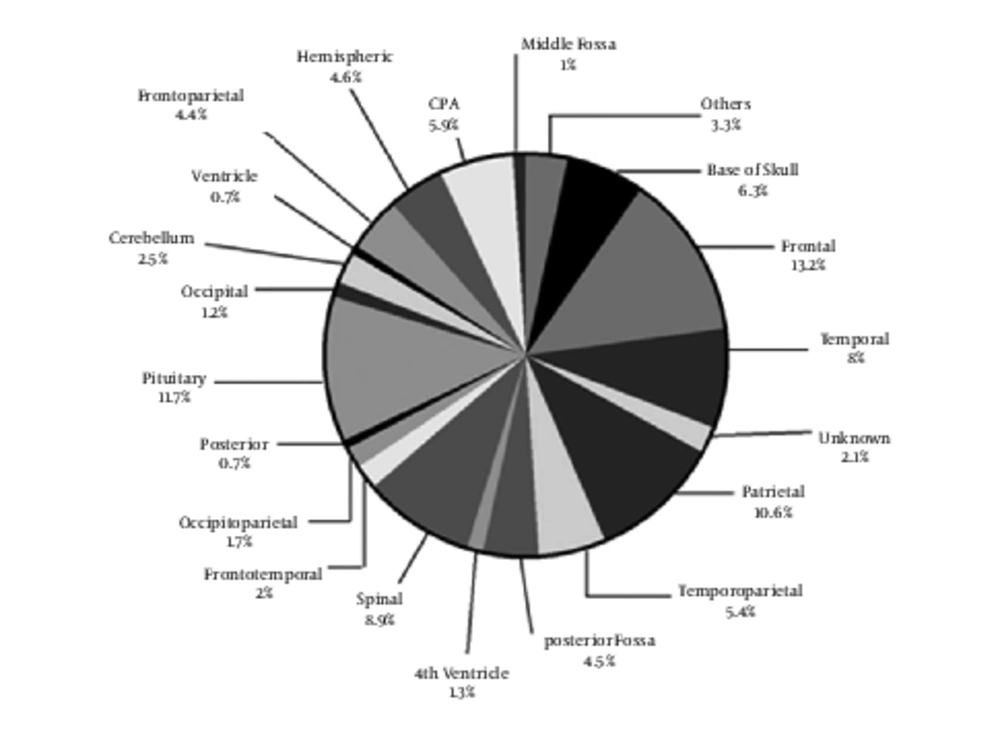
The distribution of CNS tumors by site is presented in Figure 1. The largest proportion of CNS tumors is supratentorial, developing in the frontal, temporal, and parietal lobes. Analysis shows that the most common tumor site is frontal (13.2%). Approximately, 10% of tumors are located in parietal and 8% in temporal. Cerebellum and ventricle account for 2.5% and 0.7% of all tumors, respectively. Pituitary consists of 11.7% of tumors. Results indicated that most of the brain and CNS tumors in ages 13 to 19 years occurred in posterior fossa. About one-fifth of reported histologies for tumor diagnosed in children (ages 2 - 12 years) are the mostly non-malignant tumor of pituitary gland.
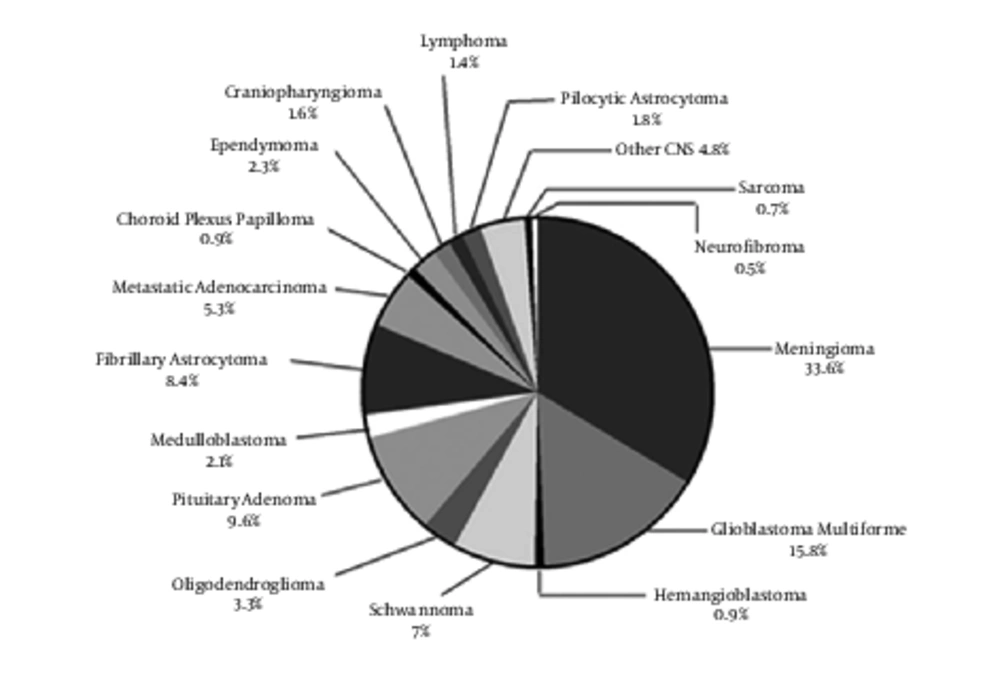
Tumors distribution by detailed histology is shown in Figure 2. The most reported histologies were predominantly non-malignant meningiomas (33.6%), glioblastomas (15.8%), pituitary adenoma (9.6%) and fibrillary astrocytomas (8.4%; including anaplastic, diffuse, and NOS), which account for more than 67% of reported tumors. Patients with glioblastomas and meningiomas had the highest mean age at the time of diagnosis. Among the age group of children and adolescent, histology of medulloblastoma had the highest number, and it decreased with advancing age. In contrast to medulloblastoma, the number of meningioma increased progressively with age. Meningiomas are uncommon in children and more common in older adults. Among 1,164 cases, rare tumors histology were including pineoblastoma, subependymal giant cell astrocytoma, hemangiopericytoma, plasmacytoma, melanoma, mixed oligoastro-cytoma, pineal paranchymal, ganglioneuroma, pilomyxoid astrocytoma, PNET, chondroidchordoma, malignant astroblastoma, pleomorphic xanthoastrocytoma, myxo-papillary ependymoma, ganglioglioma, oligoasterocytoma, atypical central neuro-cytoma, and paraganglioma considered as “others histology type” in analysis.
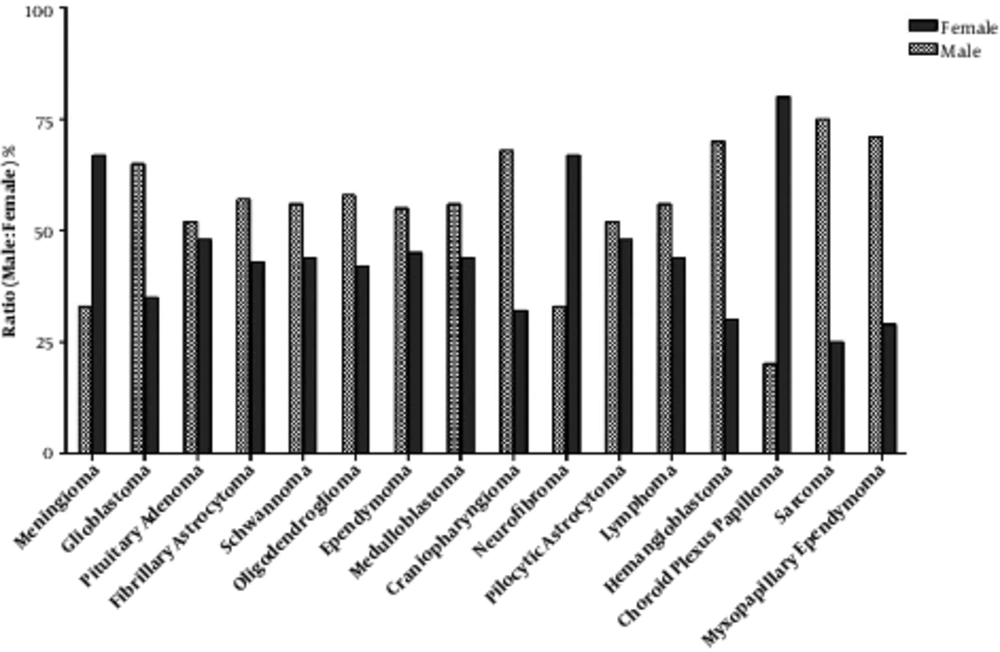
The frequency of the largest category, tumors of the meninges, was higher in female patients than in male patients (P = 0.001). Noticeably, the frequency of neurofibroma and choroid plexus papilloma tumors was higher in female patients (P = 0.03). The percent of sarcoma tumors was small (0.7%) and numbers were higher for male than female. Moreover, histologies with higher frequency in male than in female patients with the statistically significant difference included glioblastoma, Hemangioblastoma, and myxopapillary ependymoma. Sex differences were not evident for pituitary adenoma or the tumors of ependymoma. The distribution by detailed histology and sex are depicted in Figure 3.
Tumor laterality was evaluated from the collected data of surgical report among 1,057 confirmed brain tumors. The ratio of left-sided to right-sided tumors did not differ significantly for any of the major types. Moreover, brain tumors were analyzed laterality with respect to tumor grade. Low-grade meningioma and glioma non-significantly occurred in left sided, whereas, glioma grade III occurred non-significantly in right side.
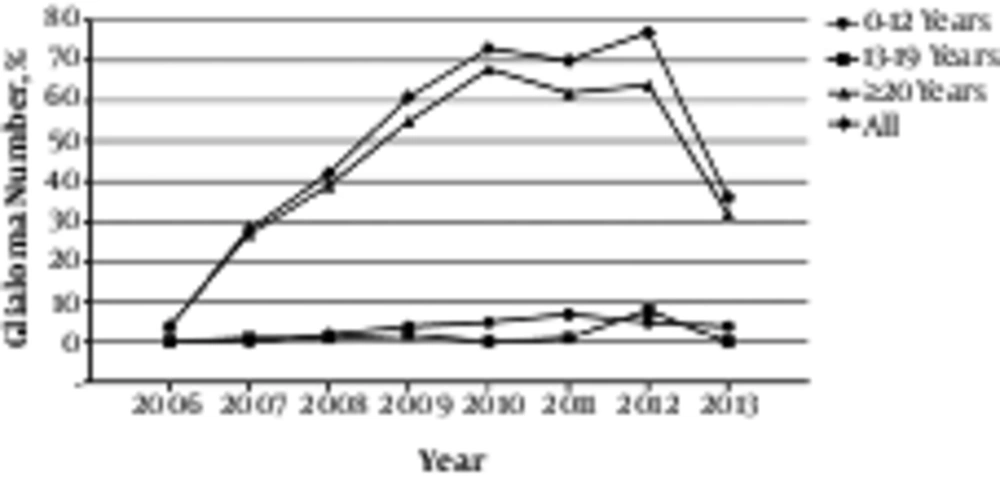
Annual incidences of all primary brain and CNS tumors from 2006 to 2013 are displayed in Figure 4. The frequency among diagnostic years 2006 to 2013 is significantly different from each year. The trend shows a sharp increment in number of CNS diagnosed tumors between 2007 until 2010. And a fluctuation can be seen in 2010 to 2012. Nevertheless, the frequency in 2013 shows a significant decrease compared with 2012.
4. Discussion
This study represents the analysis of 1,164 CNS tumors derived from a high-quality hospital record system from 2006 through 2013. This allows us to analyze, in detail, the variation frequency by age, sex, histology, site, and laterality.
Overall, the median age estimated at diagnosis for tumors of CNS was 50 years among both genders. This finding is comparable with the Europe and the United States results, in which the median age at diagnosis of CNS tumors is 53 (5) and 56 years (6), respectively. This datum did not show any significant difference in CNS frequency between males and females that is in contrast to the published data of CNS statistical report of US (7). But, for certain tumor type’s particularly significant difference was seen in gender distribution. The frequency of meningioma was almost twice greater in females than in males. This result is in consistent with other studies in Europe and US (8,9). Moreover, many studies implied the female gender as one of the most established risk factor for meningioma (10). In addition, the effect of gender on incidence of CNS tumor is almost consistent across time with the prevalence of meningioma among females (11).
Generally, the number of CNS tumor apparently increased from 2006 to 2012 and, then, leveled-off. The increase in frequency during those 6 years may contribute to progress in diagnostic technology, and also alteration in tumor classification and coding probably reflect the increase of brain tumor frequency. Regarding a decrease in the frequency of CNS tumors afterwards 2012, it seems to be necessary that future studies will have to demonstrate whether this decline continues.
The overall increase of frequency observed in CNS tumor was mainly in elderly (≥ 20 years). The frequency was almost stable in both children group (0 - 12 years) and adolescent group (13 - 19 years) over the 7-year period. Some CNS tumors express an increase in frequency in all age groups like glioblastomas, meningioma, pituitary adenoma, fibrillary astrocytomas, and schwannoma. Totally, the overall number increased with age among subjects of 50 to 60 years. Although CNS frequency increased with age, some of the CNS tumors are more common in children and younger adults. Data from various national cancer registries support variation in the epidemiology of brain tumor in adults versus children.
The results of this study show that medulloblastoma has the highest frequency of CNS tumors among children group. Mehrazin reported that astrocytoma is the commonest brain tumor in children followed by medulloblastoma (12). Also, a report from Pakistan indicated that the most common pediatric brain tumor is astrocytoma with the high frequency of pilocytic astrocytoma histology subtype in females, whereas medduloblastoma is more common in males (13). The same result from Brazil has been reported that astrocytoma is the more frequent type in pediatric CNS tumors followed by medduloblastoma (14). This is in some way different from the results of this study. On the other hand, a retrospective study in MAHAK’s Pediatric Cancer Treatment and Research Center described that medduloblastoma is the most common tumor (34.02%) (15). This finding is as same as a report in Syria, which explained that medulloblastoma ranked first in brain tumor incidence in children (16); the results of the aforementioned research is consistent with the findings of this study.
Furthermore, medduloblastoma brain tumors are known to affect children more than adults. Data from SEER database between 1973 and 2007 indicated that this type of brain tumor shows an orientation for affecting children (9.6 children per million, and 0.54 adult per million) (17).
However, there is a variation in these trends by histology and increase in incidence of some histological sub-group is obvious. The trends of high-grade gliomas and astrocytomas require more investigation.
This variation in distribution by age and by histology proposes that no single carcinogenic compound can explain this. Epidemiologic findings suggest that environmental exposure contribute to the CNS neoplasms incidence. Published studies on the effect of radiofrequency electromagnetic fields provide conflicting results that are not useful to interpret geographical differences in CNS incidence. Although the etiology of such tumors is unknown, the possible role of cellular phone could not be negligible (18). Several results did not indicate an increased risk of almost 10 years of reasonable use of mobile phone for any tumor of the brain (19). However, recent studies support association between heavy mobile phone use and brain tumors (20).
This study also presents that rare CNS tumors show an immense array of tumors with a variety of epidemiological characteristics. Although they are cited as rare tumors, the majority of them belong to different histology sub-type of astrocytic tumors. Hence, all astrocytic tumors were classified as astrocytoma in this report. Therefore, it is worth to say that the majority of epidemiological features of rare CNS tumors are influenced by astrocytic tumors. In this study, the frequency of astrocytic tumors was higher among men than women, but not significantly.
The CNS tumors incidence alters greatly across the world with the highest rate in the developed western countries (21). Besides, there is certain geographical difference in age incidence rate worldwide (22) that may be due to the availability of highly advanced imaging technology, and the use of magnetic resonance imaging results in the diagnosis of unexpected lesion.
In conclusion, although the frequency of brain neoplasms in Iran is high, only a few studies have been reported on the distribution of CNS tumors by histopathology type, age, and sex in literature. Particularly, no study has been implied on the CNS frequency in north-east of Iran. This study, which is a retrospective hospital-based rather than a population study, represents important epidemiological characteristics of CNS tumors in north-east of Iran. Hence, this data might provide a basis for further investigations.