1. Background
Borderline ovarian tumor (BOT), previously called low-grade malignant tumor, accounts for about 10% to 20% of ovarian neoplasms (1, 2). It represents increased epithelial proliferation and different degrees of nuclear atypia without distinct stromal invasion (3). Most patients with BOT are asymptomatic and may be found during routine workup (4). BOT consists of some subtypes such as mucinous, serous, endometrial, clear cell, and Brenner with mucinous and serous as the most prevalent types (1, 3). The serous type can be developed bilaterally with extra ovarian presentations with peritoneal implantation and a high rate of recurrence and malignant transformation (2, 5). A characteristic feature attributed to the mucinous tumor is the simultaneous presence of benign, borderline, and malignant neoplasms (6).
As it is diagnosed mostly in the early stage, the 5- and 10-year survival is reported to be more than 90% to 98% according to the stage of the disease. However, the recurrence rate is reported in 7% to 16% of cases (1, 2, 7). Some factors influence the recurrence development including advanced maternal age, CA125 level, and invasive implantations (3). It should be mentioned that it is controversial to attribute the prognosis and recurrence rate to the presence of micro-papillary and microinvasion patterns (8-14).
Surgery is the standard management of BOT (15). Different types of surgery may be performed as radical or fertility-sparing methods depending on age, pathology type, and the stage of disease (3, 15). Although there is controversy on the recurrence rate of fertility-sparing surgery (FSS) in BOT, the good prognosis of the tumor and the young age of patients encourage the surgeons to perform this surgery in women, who desire to preserve their fertility (2, 3, 7, 15, 16).
2. Objectives
Due to controversies about different clinicopathologic features and surgery types affecting the BOT outcomes and the probability of malignant transformation in these tumors (1, 17, 18), we aimed at investigating the relationship between clinic-pathologic features and outcomes in our center. This study aimed at explaining the controversial features present in the literature.
3. Methods
This retrospective study is approved by the Ethics Committee affiliated to Shiraz University of Medical Sciences (IR.SUMS.REC.1399.708). Medical data of all the BOT patients referred to the Motahari tumor clinic affiliated to Shiraz University of Medical Sciences from January 2010 to October 2020 were recorded. The diagnosis of BOT was made by permanent pathology, which was reported by the same gynecology oncology-specific pathologist blinded to the project. Then, the information of each patient including age (as being 40 years as cut-off) (19), histologic type of the tumor, unilateral or bilateral type, presence of micro-papillary pattern or microinvasion, tumor size (10 cm as cut-off), recurrence and malignant transformation rate, and performance of staging based on FIGO 2014 (20) and lymph node evaluation was recorded in the prepared form. Patients had 3- to 6-month interval follow-ups with pelvic examination, abdominopelvic ultrasound, and CA-125 tumor marker for 5 years. Then, they were annually followed up.
3.1. Statistical Analysis
All data were analyzed, using SPSS software version 19.0 (SPSS Inc. Chicago, IL, USA). The mean ± standard deviation was calculated for each item. Significant factors influencing the recurrence were tested, using chi-square and fisher exact test; the log-rank test and Kaplan- Meier curve were also used for survival analysis.
4. Results
4.1. Patients’ Characteristics
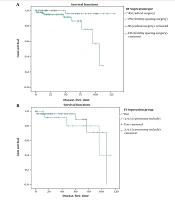
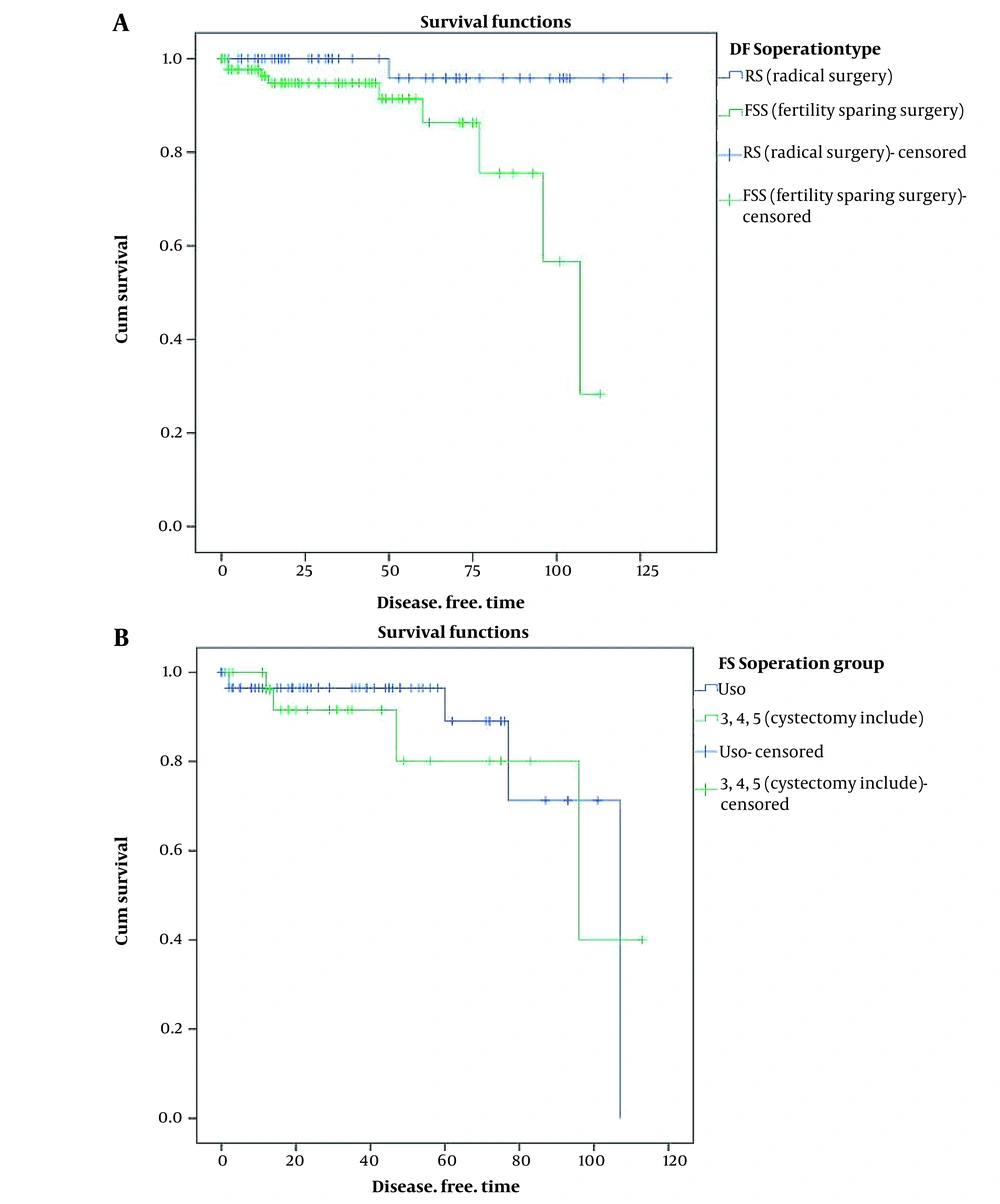
A total of 145 patients with BOTs participated in this study; their surgical data and clinicopathologic characteristics are listed in Table 1. Among all cases, 89 (61.4%) had undergone FSS, while 56 (38.6%) had radical surgery (RS). Most of the patients were diagnosed at stage IA (n = 92,63.4%), while there were 13,9% in stage IB, 11,7.6% in stage IC, 3,2.1%in stage III, and 26,17.9% had no stage. Most of the patients in the early stages desired more to preserve fertility, especially for those at stage IA (59.7% vs. 40.3%). Patients younger than 40 years old were 90, 62.1% vs. 55, and 37.9% older than 40 years. Among them, most of the patients with FSS were younger than 40 years old in comparison to the RS group (n = 81, 91% vs. n = 9, 16.1%). The tumor size of less than 10 cm was 73 (50.3%) and more than 10 cm was 63 (43.4%). The proportion of patients, who had undergone staging surgery, was higher in patients with RS (82.1% vs. 68.5%). The patients were followed up for a median of 51.5 months (range 3 - 120 months). Based on the oncological outcomes at the last follow-up, the recurrence rate in the FSS group was higher than those with RS (10.1% vs. 1.8 % P = 0.011). The Kaplan-Meier analysis of DFS is described in Figure 1A. No death was reported in each group. The difference in OS between the two groups was not significant (P = 0.328).
| Variables | Total | Fertility Sparing Surgery | Radical Surgery |
|---|---|---|---|
| Total | 145 (100) | 89 (61.4) | 56 (38.6) |
| FIGO staging | |||
| Unstaged | 26 (17.9) | 18 (20.2) | 8 (14.3) |
| IA | 92 (63.4) | 55 (59.7) | 37 (40.3) |
| IB | 13 (9) | 7 (53.8) | 6 (46.2) |
| IC1 | 11 (7.6) | 8 (72.7) | 3 (27.3) |
| II | 0 (0) | 0 (0) | 0 (0) |
| III | 3 (2.1) | 1 (33.3) | 2 (66.7) |
| Age (y) | 90 (62.1) | 81 (91) | 9 (16.1) |
| ≤ 40 | |||
| > 40 | 55 (37.9) | 8 (9) | 47 (83.9) |
| Size (cm) | 73 (50.3) | 46 (54.8) | 27 (51.9) |
| < 10 | |||
| ≥ 10 | 63 (43.4) | 38 (45.2) | 25 (48.1) |
| Localization | 133 (91.7) | 83 (93.3) | 50 (89.3) |
| Unilateral | |||
| Bilateral | 12 (8.3) | 6 (6.7) | 6 (10.7) |
| Histology | 83 (57.2) | 46 (51.7) | 37 (66.1) |
| Serous | |||
| Mucinous | 58 (40) | 39 (43.8) | 19 (33.9) |
| Others | 4 (2.8) | 4 (4.5) | 0 (0) |
| Performing staging | 107 (73.8) | 61 (68.5) | 46 (82.1) |
| Yes | |||
| No | 38 (26.2) | 28 (31.5) | 10 (17.9) |
a Values are expressed as No. (%).
Comparison of disease-free survival curves (DFS) of patients who underwent different types of surgeries (radical surgery and fertility-sparing surgery) which was significant, P: 0.011; (disease-free time is considered with month); B, Comparison of DFS curves between cystectomy and unilateral salpingo-oophorectomy, which was not significant; P: 0.631.
Serous BOT was the most common histologic type (n = 83, 57.2%) followed by mucinous (n = 58, 40%) and other types of pathology including endometrioid and clear cell (n = 4, 2.8%). Among these patients, 7 (4.8%) had micropapillary patterns, 13 (9%) had microinvasions, and 3 (1.1%) had noninvasive peritoneal implants. Unilateral BOTs were more common (n = 133, 91.7% vs. n = 12, 8.3%) than bilateral BOTs. Among 17 (11.7%) patients with lymphadenectomy, no one had lymph node metastasis. Only 8 (5.5%) patients had a laparoscopy and 137 (94.5%) underwent laparotomy. Most of the patients (n = 107, 73.8%) underwent staging surgery including peritoneal cytology and partial omentectomy with or without lymphadenectomy. The malignant transformation occurred in 2 patients, 1 with the pathology of serous borderline tumor transformed to seromucous carcinoma and the other with mucinous BOT transformed to mucinous carcinoma; 5 (3.4%) patients received adjuvant chemotherapy, 2 of whom were due to malignant transformation and 3 patients due to some pathologic characteristics (micropapillary, microinvasion) based on their physicians’ opinion.
4.2. Recurrence Outcomes of Borderline Ovarian Tumors Patients
Significant factors influencing the recurrence were tested, using chi-square, Fisher Exact test, and log-rank test survival analysis, as shown in Table 2. Among the clinicopathologic factors FIGO stage was not a significant factor for recurrence rate (9/132 in stage I vs . 1/14 in satge >I, P = 0.969). However, the recurrence interval was shorter in patients with stage ≥ IC than stage < IC (118 months vs. 91 months). Patients younger than 40 years old (≤ 40) had a significantly higher recurrence rate than > 40 (10% vs. 1.8%, P = 0.05). There was more recurrence in the tumor size < 10 cm than ≥ 10 cm (13.7% vs. 0% P = 0.013).
| Variables | Number of Recurrence Cases | % of Recurrence Cases | P-Value | Recurrence Interval (mo) |
|---|---|---|---|---|
| FIGO stage | 0.969 | |||
| Stage I | 9/132 | 22.7 | 118 | |
| > Stage I | 1/14 | 53.5 | ||
| Size (cm) | 0.013 a | N/A | ||
| < 10 | 10/73 | 13.7 | ||
| ≥ 10 | 0/63 | 0 | ||
| Histology | 0.812 | |||
| Serous | 7/83 | 8.4 | 101 | |
| Mucinous | 3/58 | 5.2 | 123 | |
| Others b | 0/4 | 0 | 114 | |
| Localization | 1 | N/A | ||
| Unilateral c | 10/133 | 7.5 | ||
| Bilateral | 0/12 | 0 | ||
| Micropapillary pattern | 0.421 | |||
| Yes | 1/7 | 14.3 | 110 | |
| No | 9/138 | 6.5 | 117 | |
| Microinvasion pattern | 0.635 | N/A | ||
| Yes | 10/132 | 7.6 | ||
| No | 0/13 | 0 | ||
| Non-invasion implant | 1 | N/A | ||
| Yes | 0/3 | 0 | ||
| No | 10/142 | 7 | ||
| Lymphadenectomy | 0.345 | |||
| Yes | 2/17 | 11.8 | 79 | |
| No | 8/128 | 6.3 | 116 | |
| Surgery approach | 0.096 | |||
| Laparoscopy | 2/8 | 25 | 37 | |
| Laparotomy | 8/137 | 5.8 | 116 | |
| Adjuvant chemotherapy | 0.421 | N/A | ||
| Yes | 0/3 | 0 | ||
| No | 10/142 | 6.3 | ||
| Staging surgery | 0.235 | |||
| Yes | 6/107 | 5.6 | 122 | |
| No | 4/38 | 10.5 | 96 | |
| Age (y) | 0.05 a | |||
| ≤ 40 | 9/90 | 10 | 100 | |
| > 40 | 1/55 | 1.8 | 128 | |
| Operation type | ||||
| Radical surgery | 1/56 | 1.8 | 0.011 a | 124 |
| Fertility sparing surgery | 9/89 | 10.1 | 93 | |
| Unilateral salpingo-oophorectomy | 5/60 | 8.3 | 0.6 | 94 |
| Cystectomy | 4/30 | 13.3 | 90 |
Abbreviations: FIGO, The International Federation of Gynecology and Obstetrics; N/A, not applicable.
a P-value ≤ 0.05 is statistically significant.
b Others included endometrioid, clear cell, seromucous.
c Unilateral included left and right.
Patients with serous BOT had a higher recurrence rate (8.4%) compared to the mucinous type (5.2%). However, the P-value was not significant (P = 0.628). In this population study, there was no recurrence in bilateral tumors; so, lateralization was not a significant factor in recurrence. None of the histological factors including micropapillary, microinvasion, and noninvasive peritoneal implants significantly influenced the recurrence rate respectively (P = 0.421, 0.635, 1.000). However, the recurrence interval was shorter in micropapillary histologies (110 months vs. 117 months). Performing lymphadenectomy was not significantly related to the recurrence rate (P = 0.345). Among the surgery approaches , laparoscopy was not a significant factor in comparision to laparatomy (25% vs.5.8% , P = 0.096); however, the recurrence interval was shorter in the laparoscopy group than in the laparotomy patients (37 months vs. 116 months). Receiving adjuvant chemotherapy was not associated with a reduction in the recurrence rate (P = 0.421). Performing staging surgery showed to have a relationship with a lower recurrence rate and longer recurrence interval; however, it was not significant. (5.6% vs. 10.5% P = 0.235), (122 months vs. 96 months), respectively. Among the participants, the patients who had undergone FSS had a significantly more recurrence rate than patients with RS (10.1% vs. 1.8%, P = 0.011). Also, the recurrence interval was shorter in the FSS group in comparison to the RS group. The patients with FSS were analyzed in 2 groups including patients who had undergone unilateral salpingo-oophorectomy and those with ovarian cystectomy. There was no significant difference in the recurrence rate between both groups; however, in the cystectomy group, there was a higher recurrence rate (8.3% vs. 13.3%, P = 0.6). The recurrence interval in these two groups was approximately similar (94 months vs, 90 months), respectively. The survival curves of different types of surgery are shown in Figure 1B.
5. Discussion
This study aimed at evaluating the clinicopathologic features of patients with BOT including age, tumor size, unilateral or bilateral lesion, type of surgery, the performance of staging or not, lymphadenectomy, presence of micropapillary or microinvasion, and malignant transformation in the patients.
One determining factor of being a low-risk group for recurrence is age less than 40 years (4). The median age of BOT is reported to be 45 years with a third of them under 40 years (7). In line with previous studies, our study revealed that patients younger than 40 years old were 62.1% vs. 37.9% older than 40 years. Among them, most of the patients, who had undergone FSS, were younger than 40 years old compared with the RS group. Tumor size less than 10cm was 50.3%, and more than 10cm was 43.4%. However, in the RS group, the proportion of the tumor is approximately the same among both groups, which can be attributed to performing FSS in patients, who seemed to be benign tumors rather than malignancies. To evaluate the recurrence, patients younger than 40 years old and with tumor size less than 10 cm were significantly associated with a higher recurrence rate. A data point is the higher rate of FSS in ages younger than 40 years and tumors less than 10 cm which justifies higher recurrence. Unilateral BOT was more common in 91.7% versus 8.3% than bilateral BOTs. There was no recurrence in bilateral tumors; so, lateralization was not a significant factor in recurrence in our study.
Among different subtypes of BOT, the highest incidence belongs to serous BOT, which is mentioned to be about 51% followed by the mucinous subtype, which accounts for about 44% in Denmark. This alteration is attributed to decreased incidence of serous type during the time (18). It is reported that the prevalence of the mucinous subtype is about 70% in Asia (1), while the serous pattern is dominant in Europe and North America (2). A contributing factor in the tumor type prevalence can be different educational levels among nationalities (18). It is mentioned that clear cell, endometrioid, and Brenner types consist of 4% to 5% of BOT (4, 14). However, in contrast to Asian reports (1), serous BOT was the most common histologic type (57.2%) followed by mucinous (40%) and other pathologies including endometrioid and clear cell (2.8%) in our center. To evaluate histologic subtypes on the recurrence, in our study, patients with serous BOT had a higher recurrence rate (8.4%) versus the mucinous type (5.2%). However, the P-value was not significant (P = 0.628). According to previous studies, there was no relationship between histological subtypes and recurrence (21). On the contrary, Chen et al. showed a better prognosis for serous tumors (6).
It is controversial to attribute the recurrence of BOT to micropapillary pattern; in this way, some authors consider prognostic effects to be related to invasive implants (1, 4, 5, 8-12, 14, 22). Despite the lack of effect on the survival rate, the micro-papillary pattern is supposed to be associated with advanced disease, bilateral involvement of the ovaries, and increased risk of peritoneal involvement consisting of lymph node involvement or microinvasion (8). Also, there is controversy on the relevance of microinvasion to recurrence, which is defined as the presence of infiltration to stroma less than 3 mm in the longest linear dimension or ≤ 10 mm2 in one or more points (14, 23). Boyraz et al. demonstrated the relevance of microinvasion to decreasing disease-free survival (13). In our study, none of the histological factors including micropapillary, microinvasion, and noninvasive peritoneal implants significantly affected the recurrence, but it was with a shorter recurrence interval in micropapillary histology (110 months vs. 117 months).
Considering the type of surgery, Morrison, Plett et al., and Li et al. believed in the importance of surgery types in recurrence (2, 16, 17, 24). In FSS, unilateral cystectomy, whether done by laparoscopy or laparotomy, is mentioned as a risk factor for recurrence (15, 22). In our study, only 8 (5.5%) patients had a laparoscopy, and 137 (94.5%) underwent laparotomy. According to the oncological outcomes at the last follow-up, performing laparoscopy was not significantly associated with a higher recurrence rate; however, the recurrence interval was shorter in comparison to laparotomy. Li et al. presented unilateral salpingo-oophorectomy, as an alternative surgery, with emphasis on laparoscopy being introduced as the preferred technique in stage I (24). It is noticeable that there are a few reports of trocar metastases in patients, for whom laparoscopy was performed besides to higher rate of recurrence and lack of accuracy in staging mentioned by some authors (4, 14). This is compatible with our study results.
Among all the cases, 89 (61.4%) had undergone FSS, while 56 (38.6%) had RS. The recurrence rate in the FSS group was higher than those in the RS group (P = 0.011). No death was reported in each group. The difference in OS between the two groups was not significant. Also, the recurrence interval was shorter in the FSS group in comparison to the RS group. We analyzed the patients with FSS in 2 groups including patients who underwent unilateral salpingo-oophorectomy and those for whom ovarian cystectomy was done. There was a higher recurrence rate in the cystectomy group, but the difference was not significant (8.3% vs. 13.3%, P = 0.6). Also, there are some apposite ideas on the role of the type of surgery on the recurrence presented in a mini-review by Maramai et al. (3). In addition, considering the survival aspect of the surgery type, FSS and RS had a similar survival rate with preserved natural fertility among the first group, despite the higher rate of recurrence in FSS (5, 25). Another encouraging point is the identical pregnancy outcome in comparison to the normal population presented by Bercow et al. in a systematic review in 2020 (26). Therefore, it is important to manage cautiously when FSS is desired according to less DFS in this type of surgery. We should consider the oncological and fertility outcome benefits of FSS in women, who desire to preserve their fertility.
As another controversy on the surgery technique, it is recommended that staging should be performed during BOT surgeries by omental and peritoneal biopsies and routine lymphadenectomy should be avoided since even lymph node involvement may not alter the survival and recurrence rate of the disease (7, 14, 27); however, some authors do not agree with the benefits of staging (24). Yilmaz et al. presented involvement of lymph nodes in 25% of cases with a lack of effect on survival, which is in contrast to previous studies (4, 14). In our study, most of the patients (73.8%) underwent staging surgery including peritoneal cytology and partial omentectomy with or without lymphadenectomy, which was higher in patients with RS. Performing staging surgery was associated with a lower recurrence rate and longer recurrence interval, but the difference was not significant. However, none of the patients with lymphadenectomy had lymph node metastasis, and it was not significantly related to the recurrence rate. The evaluation of the recurrence rate according to the disease stage showed no significant difference between stages IA + IB and stages more than IC, which is due to the low malignant potential nature of these tumors. Moreover, receiving adjuvant chemotherapy was not associated with a reduced recurrence rate. The risk of malignant transformation is still not clear. It is believed that BOTs can progress to low-grade serous. Recurrence, as an invasive carcinoma in BOTs, may be the result of a true progression or de novo development of ovarian carcinoma (28). In our study, 2 patients developed malignant transformation, 1 with the pathology of serous BOT at stage IC, which developed to seromucous carcinoma with a recurrence interval of 50 months, and the other one progressed from mucinous BOT to mucinous carcinoma at stage III with a disease-free period of 14 months. Both patients had undergone FSS (unilateral salpingo-oophorectomy).
The strength of our study was that it was conducted on a large population who referred to the main referral center with a variety of patient characteristics. Also, we studied both aspects of the patient’s features and recurrence outcomes of BOT. The limitation of our study was the natural variation of the features among various nationalities.
5.1. Conclusions
BOT is a challenging tumor that is most prevalent in young women that desire to have fertility with questionable characteristics of recurrence in different studies reported so far. FSS and higher FIGO stages are associated with more recurrence rate in BOT patients. Although micropapillary and microinvasion were not significantly related to higher recurrence rates in our study, they are challenging issues in BOT among different studies. Due to the high prevalence of BOT among young women and the desire for fertility preservation, we should pay more attention to clinicopathologic and surgery types affecting the recurrence of BOTs and consider oncological and fertility benefits. Consequently, it is necessary to perform prospective studies in larger populations to evaluate clinicopathological and surgery types affecting oncological outcomes, especially with longer follow-up periods.