1. Background
Squamous cell carcinoma (SCC) accounts for over 90% of head and neck cancers (1, 2). In the United States, the prevalence of oral cancer is 3% and 2% in males and females, respectively (3). Around 27 000 new cases are diagnosed with cancer annually, 5 500 of whom die (4). Evidence shows that potentially malignant disorders (PMDs) are associated with the development of malignant lesions. PMDs are defined as transformed lesions with a risk of malignant transformation higher than that of normal tissue (5). The potentially malignant conditions refer to diseases or conditions that do not necessarily change the appearance of tissue but have a higher risk of transformation to a premalignant lesion or cancer. Leukoplakia, erythroplakia, submucosal fibrosis, actinic cheilitis, and erosive lichen planus are among the pharyngeal, laryngeal, and oral potentially malignant disorders (OPMDs) (5). According to the definition by the World Health Organization, leukoplakia is a white patch, which cannot be clinically or histopathologically categorized under any other disease category. Although leukoplakia does not have a distinct histopathological diagnosis, it is considered an OPMD and has a risk of malignant transformation higher than that of normal tissue. On the other hand, it is the most common OPMD and accounts for 85% of OPMDs. Also, over one-third of oral cancerous lesions have an adjacent leukoplakia (6). Evidence shows that the global prevalence rate of leukoplakia is 1.5% to 4.3%, and it has a higher prevalence in males (7). On the other hand, in the past 50 years, a reduction has occurred in the number of affected males, which is probably attributed to improved diagnosis and enhanced knowledge of the medical personnel. Dysplasia or carcinoma in situ only occurs in 5% to 25% of leukoplakia cases. It has a recurrence rate of 10% to 35% after surgical removal. The possibility of the development of a new lesion also exists (8). In particular, the granular and verrucous lesions have an 83% possibility of recurrence, necessitating a second surgical procedure (9). Nonetheless, the risk of the development of malignancy even after surgery is unclear (10). Evidence and statistics regarding the rate of malignant transformation of leukoplakia are variable and inconclusive in the literature. However, it has been estimated that around 2% of leukoplakia lesions undergo cancerous transformation annually. Also, it has been reported that malignant transformation occurs 2 to 4 years after the initial development of the lesion; although it may take months or decades in some cases. The malignant transformation potential of leukoplakia depends on the rate of dysplasia. Lesions with moderate or severe dysplasia have a 4% to 11%, and 20% to 43% risk of malignant transformation, respectively (11). Although leukoplakia lesions that are not associated with dysplasia are not surgically removed, they require regular follow-ups due to the possibility of their progression. Thus, long-term follow-ups and examinations are imperative (12).
Erythroplakia is another OPMD. Similar to leukoplakia, erythroplakia cannot be clinically or histologically categorized under the category of any other disease. Almost all cases of erythroplakia show significant dysplastic changes, carcinoma in situ, or SCC.
The point prevalence rate of erythroplakia (the number of patients with an active lesion at a certain time point) in the oral cavity has been estimated to be 1 per 2500 adults. Its exact incidence rate is unclear. However, in the United States, the prevalence of carcinoma in situ, which is more common than erythroplakia, is reportedly 1.2 per 100000 population. Although the prevalence of erythroplakia is lower than that of leukoplakia, it has a significantly higher potential for severe dysplasia at the time of biopsy or later in the future (13). Collection, registration, and analysis of the data regarding new cancer cases are the cornerstones of cancer control programs (prevention, screening, early diagnosis, and treatment). A cancer registry is the main tool for cancer management and control. It is imperative not only for epidemiological studies, but also for strategy planning, event prediction, and assessment of the accuracy of studies and efficacy of interventions. In other words, effective screening programs and assessment of the efficacy of research projects and preventive approaches are only possible by using the data available in cancer registry databanks. Thus, control services cannot be implemented without having a comprehensive cancer registry.
The decision regarding the establishment of a cancer registry system in Iran was made after the ratification of the “mandatory registration and reporting of cancer cases” bill in the Iranian parliament in 1984. Its implementation program was designed by the Cancer Control Organization in 1986. The first report regarding cancer registration in Iran was published in 1986 by registering around 18435 cancer cases (14). According to the existing literature, some registration systems are now available for vulvar and vaginal precancerous lesions (15). Early detection of patients with OPMDs and their registration in the OPMD registry enables their targeted systematic monitoring and effective management. The OPMD registry can also provide valuable information regarding the epidemiology of such lesions. Moreover, early intervention and prevention of the progression of these lesions and their malignant transformation can greatly decrease the adverse consequences of cancer and the high costs of treatment. To the best of the authors’ knowledge, there is no registration system or center for registry and follow-up of OPMDs neither in Iran nor in other countries (16).
2. Objectives
This registry aimed at introducing an exact data gathering method for detecting, managing, and monitoring patients with OPMD to decrease the occurrence of oral cancer.
3. Methods
Shahid Beheshti Dental School is an educational-therapeutic center that provides dental care services to patients. Of patients presenting to the dental clinics of the university, those diagnosed with any OPMD are referred to oral medicine specialists for a definite diagnosis. After confirmation of the diagnosis, and upon approval of the specialist regarding the eligibility of patients for inclusion in this study, the patients were briefed about the study objectives and protocols. Patients who agreed to participate signed informed consent forms and their information was uploaded to the respective software. This research project was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.DRC.REC.1398.180) and the research council of the university (code: 17253). In addition, the quality assurance proposal of this project was suggested to the research council on 10.31.2020 and approved by the ethics committee on 11.2.2020 (IR.SBMU.DRC.REC.1399.058). Details about quality control checklist items are attached in the Supplementary File. This registry was already approved by Research and Technology Deputy, Ministry of Health, Treatment, and Medical Education in 2019.
To ensure the quality of the data registry, the following issues were considered: (1) the variables of the registry were set according to the objectives; (2) A comprehensive data dictionary was defined; (3) several meetings were held to determine the minimum required variables; (4) surveillance of patients was planned following an appropriate flowchart designed for this purpose; (5) the executors of the registry applied advanced supervision on filling out the data form and variables; (6) several workshops were held to train partner centers regarding how to fill data forms and how to manage and follow the patients; (7) an executive manual protocol of registry was written; (8) the age, gender, place of birth, place of residence, level of education, occupational status, risk factors, personal and lifestyle habits, medical history, pharmaceutical history, blood type, location of the lesion, the morphology of lesion, duration of a lesion in the oral cavity, vital staining of tissue and its results (toluidine blue), symptoms, color of the lesion, clinical diagnosis, histopathological diagnosis, medical and therapeutic measures, change in the lesion in the follow-ups (presence/absence), change in the size of the lesion, change in personal and lifestyle habits, and risk factors were all recorded. The recorded details for each case are summarized in Table 1.
| Variables | Definition |
|---|---|
| Age | Day, month and year of birth |
| Gender | Male, female, unknown |
| Occupation | Self-employed, staffer, governmental-employed, work in home, unemployed, others |
| Tribe | Fars, Turkish, Kurdish, Lurs, Arabian, Baloch, Gilaks, others |
| State/city/address | Birth - last five years |
| Nationality and patient ID | Iran - unknown |
| Phone number | Cell phone/phone |
| Educational level | University degree, high school diploma, under diploma, illiterate |
| Risk factors | Smoking, alcohol consumption, hookah consumption, chewable tobacco, illegal substances (opioids, methamphetamine, heroin) |
| Location of lesion | Labial mucosa, tongue, gingiva, buccal mucosa, retromolar pad, palatal mucosa, tuberosity, vestibular mucosa, alveolar mucosa, floor of the mouth |
| Morphology of lesion | Macule, papule, patch, plaque, erosion, ulcer, Wickham's striae, others |
| Duration of the lesion | Duration of the presence of a lesion (in months) |
| Size | The largest diameter of the lesion |
| Blood type | A+ , A- , B+ , B- , O+ , O- , AB+ , AB- |
| Vital staining (toluidine blue) | Not performed – performed (positive result or negative result) |
| Symptoms | Without symptoms, pain, burning sensation, roughness, hemorrhage, others |
| Color | Red, white, red & white, pigmented, others |
| Provisional and final diagnoses | Homogenous leukoplakia, non-homogenous leukoplakia (speckled, verrucous, nodular), PVL, erythroplakia, lichen planus (erosive, plaque-type, ulcerative), submucous fibrosis, discoid lupus erythematosus, epidermolysis bullosa, Vivadent leukoplakia, verruciform xanthoma, Candida leukoplakia, graft-versus-host-disease, reverse smokings’ palate, verrucous hyperplasia, xeroderma pigmentosum, oral verrucous carcinoma, syphilis (third stage), dyskeratosis congenita, Plummer-Vinson syndrome, malnutrition, vitamin A, B, C deficiency, immunosuppressive diseases (AIDS) |
| Histopathological diagnosis | Epithelial hyperplasia, dysplasia (mild, moderate, severe), lichen planus, lichen planus plus dysplasia, carcinoma in situ, lichenoid dysplasia, SCC, micro-invasive SCC |
| Therapeutic measures | Follow-up (3 months, 6 months, annually), incisional biopsy, excisional biopsy, pharmaceutical therapy |
| Follow-up | Follow-up frequency, follow-up dates, the status of the lesion (evidence [previous lesion, new lesion, recurrent lesion] or no evidence, changes in size, location, and morphology), elimination of risk factors, post-follow-up treatment |
Data were collected by questioning the patients, clinical examination by specialists, and histopathological findings. Data were, then, recorded in the registry system.
The registry software is web-based. This software operates responsively independent of the data input tool. Aside from local use in Shahid Beheshti Dental School, it can be used for setting up a regional registry with slight modifications (accessing protocols). This software can connect between partner centers in different cities of Iran. In addition, different access levels are provided for the project manager and other users of this software.
In general, the functions of the OPMD registry software include case finding, abstracting, follow-up, quality control, and reporting, in an orderly manner based on the standards for the registry of diseases (17, 18).
Case finding: The inclusion criterion was patients with a clinically or histopathologically confirmed diagnosis of OPMD. The exclusion criteria were: (1) cases with lesions similar to OPMDs but with a final histopathological diagnosis other than OPMD; and (2) patients not consenting to record their information in the registry system.
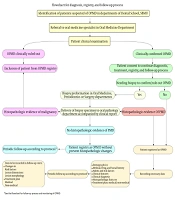
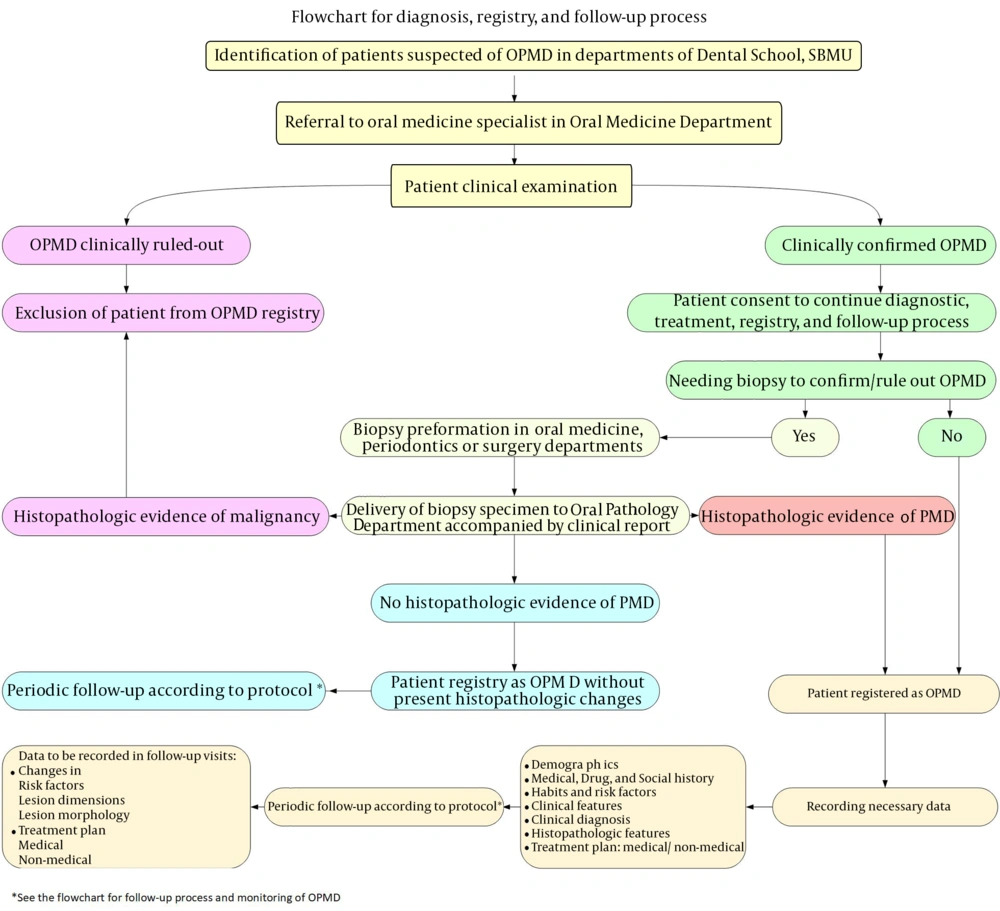
The process of detection, registration, and monitoring of patients according to Figure 1 is as follows: In case of detection of patients suspected of OPMDs in different university departments, they are referred to oral medicine specialists at the Oral Medicine Department of the university. The patients are examined and if the lesion is not confirmed to be an OPMD, the patient would not be registered in the system. If the suspected lesion is confirmed to be an OPMD, the patient is scheduled for the diagnostic work-up, treatment, registry, and monitoring processes. If the patient does not consent to registration, he/she would not be registered in the system. The patients who consent to registration would go through the diagnostic work-up. The information of patients who have sufficient clinical evidence for the diagnosis of OPMD is directly uploaded to the registry system. Cases that require biopsy for definite diagnosis undergo biopsy in the Oral Medicine, Periodontology, or Oral and Maxillofacial Surgery Departments. The biopsy specimen along with the suspected clinical diagnosis is sent to the Oral and Maxillofacial Pathology Department of the university. Pathologists assess the biopsy specimen for dysplastic changes. If such changes are confirmed, patient information as a case of OPMD is uploaded to the registry system. If the biopsy specimen is diagnosed as a malignant lesion (passing the pre-malignancy phase), the patient is not registered in the system. If the lesion does not show dysplastic changes, the lesion is registered as an OPMD that currently has no dysplastic changes. These patients are scheduled for periodic follow-ups according to the protocol to detect initiation of dysplastic changes in the old lesion or recurrence. In case of confirming microscopic dysplastic changes, the patient information is registered in the system as a confirmed case of OPMD. Personal and demographic information, medical history, pharmaceutical history, risk factors, the clinical manifestation of the lesion(s), provisional diagnosis, histopathological diagnosis, final diagnosis, treatment plan, and pharmaceutical/non-pharmaceutical therapy are all recorded in the system. Next, the patients are scheduled for periodic follow-ups, which include recording the changes in risk factors, changes in the size of the lesion, changes in the morphology of the lesion, and new diagnostic and therapeutic requirements such as new biopsy or tissue staining. Accordingly, detection, registration, and monitoring of patients with OPMDs can be performed.
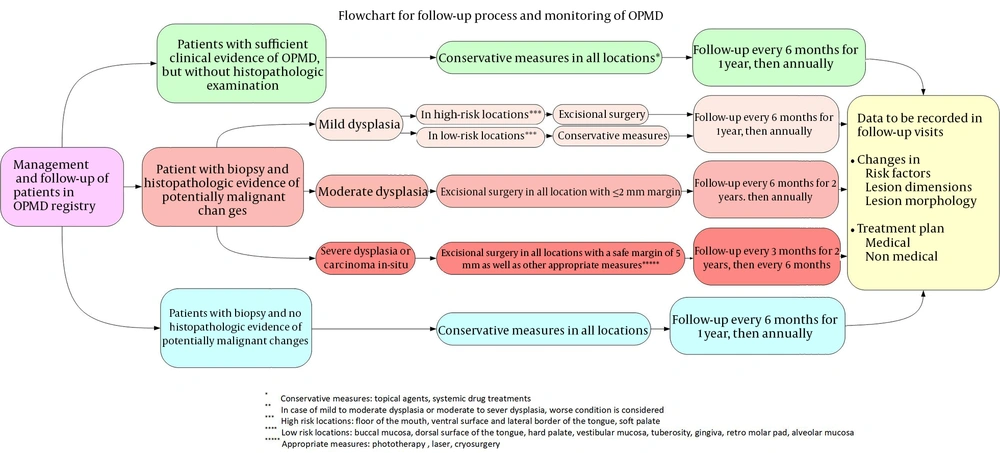
The follow-up process and monitoring of patients with OPMDs are shown in Figure 2. Patients are classified into 3 categories: (1) the first group consists of patients who have sufficient clinical evidence of OPMD and for whom no biopsy has been performed; (2) the second group consists of patients for whom biopsy has been performed and there is histopathological evidence of dysplastic changes; (3) the third group is patients for whom biopsy has been performed and there is no histopathological evidence of dysplastic changes. Figure 2 shows the details of how decisions are made about treatment and patient recall schedules in each of these groups.
Follow-up process and monitoring of OPMD patients (19)
In general, long-term follow-up of patients with OPMDs has been recommended. The interval between the follow-up sessions for leukoplakia has been variable in different studies, ranging from a couple of months to 1 year (20). Some researchers have recommended close follow-up of patients every 3 to 6 months, associated with cessation of tobacco use and alcohol use, and having a nutritional regimen rich in vegetables and fresh fruits (21). Dysplastic degree of OPMDs and involved anatomical locations are two important factors considered in decision-making regarding the follow-up protocol. It has been stated that patients who remain disease-free for up to 3 years after treatment of OPMD do not require further follow-ups. Follow-ups at 6-month intervals have been recommended for lesions such as lichen planus, leukoplakia, erythroleukoplakia, and erythroplakia without dysplasia. Follow-ups at 3-month intervals have been recommended for lesions with mild to moderate dysplasia. Monthly follow-ups have been suggested for cases with severe dysplasia or carcinoma in situ (22).
Awadallah et al. recommended conservative management and follow-ups scheduled every 6 months in the first year, and annually thereafter for patients with mild dysplasia. They suggested excisional surgery with a 2 mm safe margin for cases of moderate dysplasia followed by follow-ups scheduled every 6 months in the first 2 years and then annually. For patients with severe dysplasia and carcinoma in situ, they recommended excisional surgery with a 5 mm safe margin followed by follow-ups every 3 months in the first 2 years and then every 6 months (19). The follow-up protocol of patients in our designed OPMD registry system is similar to the protocol suggested by Awadallah et al. with more details in Figure 2.
This registry is getting the advantage of being supervised and sponsored by the Disease Registry Center of Shahid Beheshti University of Medical Sciences.
4. Results
The following steps have been accomplished so far: (1) development of an ethically-approved proposal; (2) development of a comprehensive data form; (3) development of a web-based registration software with ongoing reevaluation and improvement stages; (4) signing a contract with the Research Deputy of Shahid Beheshti University of Medical Sciences; (5)codification of a verified and ethically approved quality assurance proposal; (6) signing a collaborative contract with Tehran, Birjand, Babol, Semnan, Zahedan, and Alborz dental schools with some other dental schools such as Ilam and Shahrekord joining us; (7) presenting a lecture under the title of: “Introducing an innovative registry system for Oral Potentially Malignant Disorders (OPMD)” at the 16th Annual Congress of International Association of Dental Research (IADR), Iranian Division, 24 - 25 December 2020; (8) acquiring an acceptable score (682 out of 1000) during the first assessment of quality control (Nov 2020) was higher than the mean score (605) of other registries of SBMU; (9) holding a 3-hour webinar under the name of “Oral Potentially Malignant Disorders of the Oral Cavity: Diagnosis, Management, and Surveillance of Patients” held by the Iranian General Dentists Association in July 2021; (10) four virtual workshops: "Training on registration and monitoring of patients with pre-malignant disorders (OPMD) and how to complete the data form" were held; (11) compilation of 3 proposals adapted from the OPMD registry; (12) a total of 100 patients with OPMDs were detected from 2.2.2019 to 3.17.2021 and the checklist of registry of OPMDs was filled out for them.
Of detected patients, 46 were males and 54 were females. The mean age was 54.3 years for females and 52.3 years for males. The distribution of the type of detected OPMDs and gender of registered patients is presented in Table 2. Out of 100 patients enrolled in this project, histopathological evaluation was performed for 23 patients. Degrees of cellular changes to malignancy (cell dysplasia) were observed in 18 cases and 5 cases had no dysplasia.
| OPMD and Oral Lesion | Male | Female |
|---|---|---|
| Leukoplakia (number: 19) | ||
| Leukoplakia ( non-homogenous " speckled") | 2 | 1 |
| Leukoplakia ( non-homogenous " verrucous") | 1 | 2 |
| Leukoplakia ( non-homogenous "PVL") | 2 | 2 |
| Leukoplakia ( non-homogenous "speckled", verrucous") | 1 | 0 |
| Leukoplakia (homogenous) | 6 | 2 |
| Oral lichenoid reactions (number: 79) | ||
| Lichen planus (erosive) | 11 | 20 |
| Lichen Planus (plaque type) | 9 | 3 |
| Lichen Planus (reticular) | 1 | 5 |
| Lichen Planus (reticular-erosive) | 3 | 3 |
| Lichen Planus (reticular-erosive-ulcerative) | 1 | 0 |
| Lichen planus (ulcerative) | 6 | 5 |
| Lichen Planus (erosive-ulcerative) | 0 | 2 |
| Lichen planus (ulcerative, erosive, plaque-type) | 0 | 1 |
| Lichen Planus (reticular-ulcerative) | 0 | 1 |
| Lichen Planus (reticular-plaque type) | 0 | 1 |
| Lichen planus (erosive, plaque type) | 0 | 3 |
| Lichen Planus (ulcerative, plaque type) | 0 | 1 |
| Drug-Induced Lichenoid Reaction | 0 | 1 |
| Lichenoid Contact Reaction | 0 | 1 |
| Graft versus host disease | 1 | 0 |
| Discoid lupus erythematosus (number: 1) | 1 | 0 |
| Others (Traumatic Ulcer) (number: 1) | 1 | 0 |
| Total: 100 | 46 | 54 |
5. Discussion
The strengths of this study were as follows: relatively complete data form, definite histopathological diagnosis, patient follow-up at certain time points, using a software program that can be linked to the national registry system, cooperation with related departments such as periodontics, oral and maxillofacial surgery and oral pathology departments for patient referral and helping the process of diagnosis and treatment, having a quality control proposal for qualitative monitoring of entry and registration of information.
The limitations and weaknesses of this study were as follows: limited coverage of all patients with OPMDs in Tehran and other cities of Iran, which will be eliminated or minimized in the next phases of the project following the signing of an agreement with other dental schools in Tehran and other cities. Up to now, collaborative contracts have been signed with Tehran, Birjand, Babol, Semnan, Alborz, and Zahedan dental schools with some other dental schools such as Ilam and Shahrekord being joining us.
Numerous factors are influential in causing malignant and pre-malignant disorders of the oral cavity, such as lifestyle, social and cultural habits, and beliefs of different ethnic groups (23). Collaborative dental schools in this project are located in various geographical locations in Iran. Recording patients' information in all parts of Iran can be effective in identifying risk factors, epidemiological, clinical, and histopathological characteristics of precancerous oral lesions and comparing them with each other. Tehran Dental School, the oldest dental school in Iran, is the referral center of many cities in Iran. Zahedan dental school is geographically located in an area, where habits predisposing to pre-malignant disorders are common.
The registry data are saved in a database. The database and the archives are backed up daily. Also, to ensure optimal performance, availability, and security of the OPMD registry software, its server has been located in the IT center of SBMU Dental School.
For data distribution in the performance process of this registry, a dashboard and report section have been designed in the registry software. The dashboard includes: (1) appropriate and up-to-date diagrams and charts; (2) printable output; (3) outputs with different formats for independent analysis of the data using statistical software programs; and (4) analytical reports. The registry data are pure, and analytical reports are provided to the beneficiaries of the registry system and those that have been granted access to the registry data. The beneficiaries of this registry system include group managers, mentors and instructors, and students from the oral and maxillofacial surgery, oral pathology, and oral medicine departments. Moreover, future research projects can be planned by using the OPMD registry data with machine learning and artificial intelligence algorithms for the diagnosis and prediction of cancer. In conclusion, the prospect of this registry is to provide patients with OPMD with a nationwide diagnostic service and continuous monitoring protocol through a collaborative network of all Dental schools in Iran.