1. Background
Oral lichen planus (OLP) is a relatively common chronic inflammatory autoimmune disorder, whereby cytotoxic T lymphocytes are activated against epithelial cells. From a histological point of view, OLP is characterized by the high infiltration of T lymphocytes in the subepithelial area and the degeneration of basal keratinocytes (1, 2).
OLP largely involves inflammation-related cytokines generated by some immune and specific nonimmune cells, including keratinocytes, endothelial cells, and fibroblasts (3). OLP is more resistant to treatment than its cutaneous counterpart with potential for malignant transformation into oral squamous cell carcinoma (OSCC) (1). A large number of early-stage OSCCs clinically appear as premalignant or potentially malignant oral lesions (PMOL) (4, 5). World Health Organization (WHO) has identified lichen planus as a PMOL with a risk of transforming into malignancies (1). Therefore, early diagnosis of OLP is critical. Using biomarkers to evaluate OLP in the early stages can improve preventive therapies to suppress the disease in its initial phase (6).
Transforming growth factor-β (TGF-β) as a marker associated with inflammatory processes in chronic inflammatory conditions consists of structurally related molecules engaged in cell functions including differentiation, proliferation, motility, extracellular matrix production, angiogenesis, apoptosis, and tumorigenesis (7).
Human TGF-β has 3 isoforms, namely TGF-β1, -β2, and -β3, of which TGF-β1 is the most common. Different genes encode such isoforms. For example, TGF-β1 is encoded on chromosome 19q13 (7, 8). A variety of cells produce TGF-β1 which includes both immune and nonimmune cells, epithelial cells, and fibroblasts. TGF-β1 is responsible for a wide range of functions such as tissue regeneration and the immune response (9).
This marker as one of the most important cytokines also plays a pivotal role in the proliferation and differentiation of cancerous cells and their progression and metastasis into cancer during the epithelial-mesenchymal transition (EMT). EMT is essential for numerous physiological processes such as fetus development, fibrosis, and wound healing. It is also a fundamental factor for the cancer initiation and transition to carcinoma. It has been reported that EMT plays an important role in developing a malignant phenotype (10, 11). During EMT, TGF-β affects epithelial cells, reduces the expression of cell-adhesion proteins such as cadherin, and increases expression of mesenchymal proteins such as vimentin, and paves the way for cell migration, and invasion. Such a process can be induced by several growth factors such as TGF-β, TGF-α, EGF, and the like (10-12).
2. Objectives
Few studies have examined the expression of TGF-β1 in OLP (13, 14). Therefore, in the current study, we investigated the pathogenesis of OLP by examining the expression of TGF-β1 in epithelial cells and sub-epithelial lymphocytes of non-dysplastic OLP as a chronic inflammatory condition and compared it to dysplastic OLP as a precancerous lesion with a greater potential for transforming into OSCC.
3. Methods
A total of 40 samples from 15 dysplastic (moderate dysplasia) and 15 non-dysplastic OLP patients as well as 10 normal oral mucosa of healthy individuals were assessed by immunohistochemistry (IHC) (15). Ethical approval for this study was provided by the Ethical Committee of Mashhad University of Medical Sciences (with the code number: IR.MUMS.DENTISTRY.REC.941395.).
Patients with erosive/atrophic forms of OLP referred to the Department of Oral Medicine, School of Dentistry, Mashhad University of Medical Sciences were included in this study. Samples that were confirmed both clinically and histopathologically according to the WHO guidelines were selected (1, 16). Inclusion criteria were the presence of bilateral white striations or papules with or without atrophic/erosive lesion, and band-like lymphocyte infiltration with signs of degenerative liquefaction of the basal cell layer (12). Subjects with a history of coexisting oral premalignant lesion or oral tumor, presence of systemic illness, and history of having systemic illness were excluded.
The tissues were sectioned and histologically assessed after hematoxylin and eosin staining. Histological examinations were confirmed by 2 separate examiners. The hematoxylin- and eosin-stained sections were examined under 10× and 400× magnifications to assess dysplastic features. IHC was applied on 4 µm formalin-fixed and paraffin-embedded tissue sections. The histological slides were deparaffinized in xylene, rehydrated by a series of ethanol solutions with descending concentrations. To retrieve antigen, the sections were placed in a 0.01citrate solution (pH = 6.0) for 10 minutes. A solution of methanol with 0.5% hydrogen peroxide was used for 10 minutes to block endogenous peroxidase activity. The tissue slides were then washed in Tris-buffered saline (TBS) pH 7.6 and incubated for 10 minutes with diluted normal serum. Next, the monoclonal antibody anti-TGF-β (Novocastra Laboratories, UK, 1:50 dilution) was used at room temperature for 30 minutes.
The cytoplasmic expression of TGF-β in epithelial cells and subepithelial lymphocytes was examined by IHC. After examining 100 cells using a light microscope (Labomed Inc., USA) with 400× magnification and in 5 microscopic fields in hotspots (zones with the highest density of cells), the immunoreactivity of epithelial cells and subepithelial lymphocytes was assessed and labeled according to the following labeling index(LI); (−) no epithelial cell with cytoplasmic staining, (+1) percentage of stained cells was 20% or less, (+2) percentage of stained cells was20-50%, and (+3) percentage of stained cells was 50 - 100% (7). Each TGF-β –stained slide was evaluated by 2 researchers as independent observers.
The data were analyzed using SPSS version 22.0. Kruskal-Wallis and chi-squared tests were used to assess the statistical differences in the groups studied. The significance level was considered to be P < 0.05.
4. Results
Forty samples (dysplastic OLP, n = 15; non-dysplastic OLP, n = 12; normal oral mucosa, n = 10) were evaluated in this study. There were no significant differences between the groups in terms of age and gender.
4.1. TGF-B Expression in the Epithelium of the Studied Groups
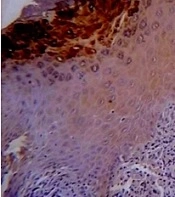
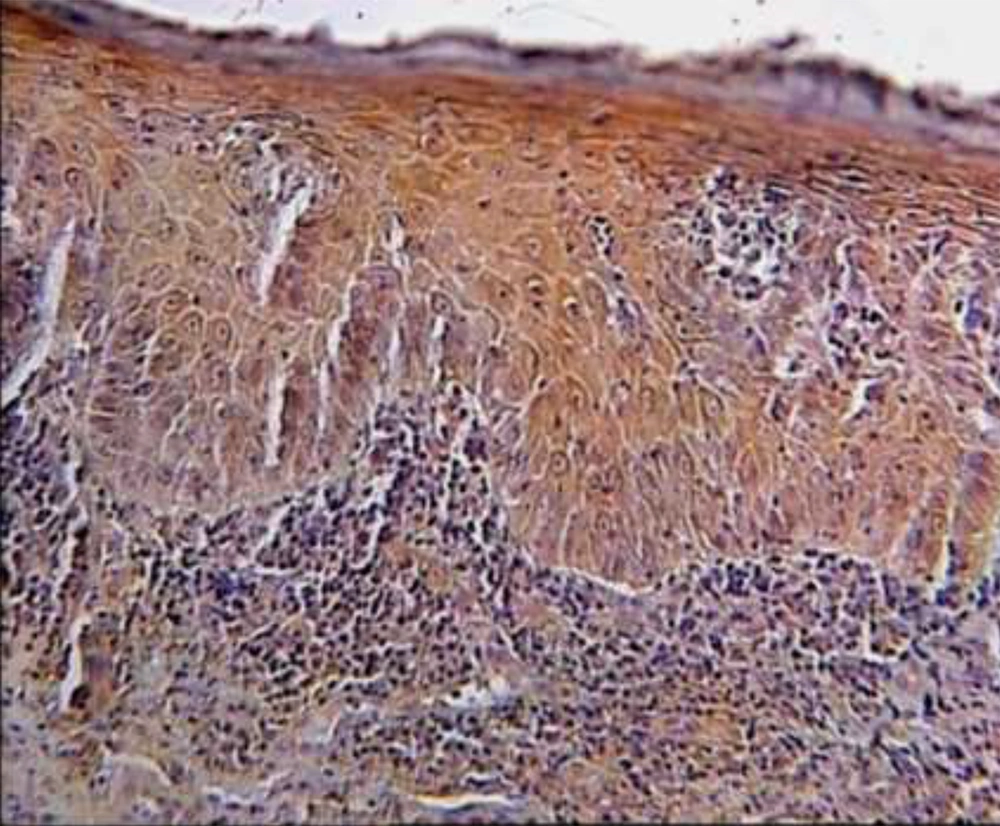
TGF-B was expressed with different degrees in all lining epithelial cells of OLPs, whereas a negative epithelial immunoreactivity was observed in 20% of normal mucosa samples. Figure 1 shows the representative staining and the data are given in Table 1.
| Lesion | TGF-LI, No. (%) | Mean Total Score ± SD | |||
|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | ||
| Normal mucosa (n = 10) | 2 (20) | 8 (80) | 0 | 0 | 0.80 ± 0.42 |
| Non-dysplasticOLP (n = 15) | 0 | 0 | 6 (40) | 9 (60) | 2.60 ± 0.51 |
| Dysplastic OLP (n = 15) | 0 | 0 | 2 (13) | 13 (87) | 2.87 ± 0.35 |
| P-value | < 0.001 | ||||
Abbreviations: SD, standard deviation; OLP, oral lichen planus
A pairwise comparison of TGF-B expression was applied in the studied groups. Statistically, significant difference was observed between dysplastic and non-dysplastic groups as well as dysplastic and normal groups (P < 0.001). A significant difference was not reported between normal and non-dysplastic groups (P = 0.116).
4.2. TGF-B Expression in the Subepithelial Lymphocytes of the Studied Groups
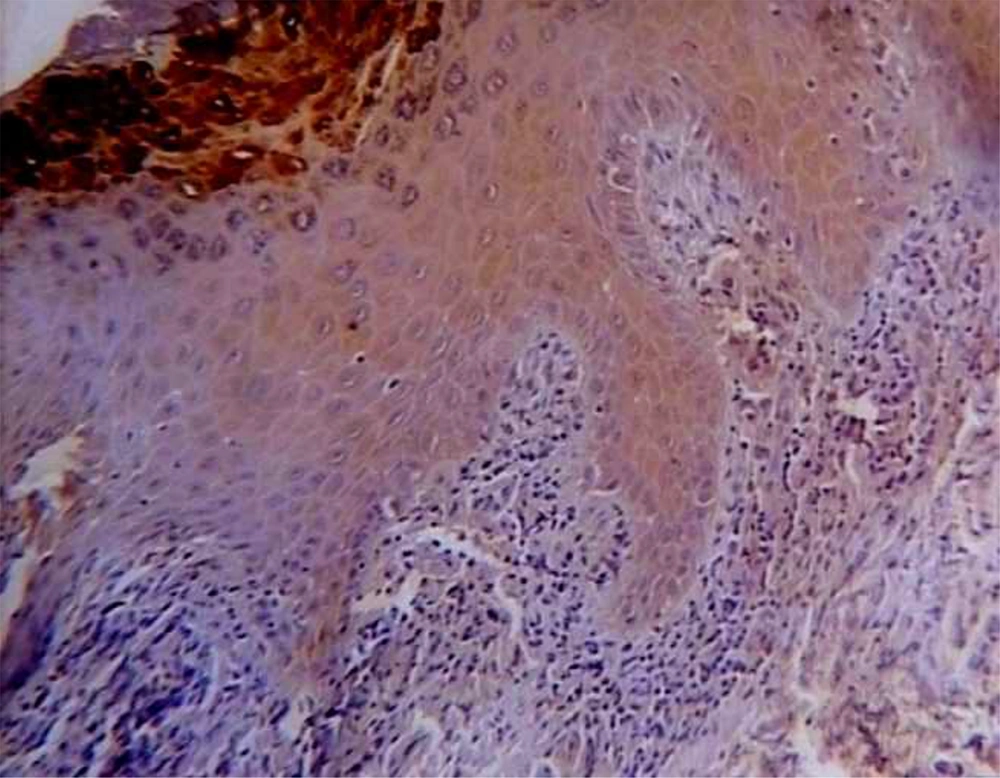
Eight samples of non-dysplastic OLPs showed negative immunoreactivity while all samples of dysplastic OLPs demonstrated 2+ and 3+ LI. There was a statistically significant difference in the studied groups (P = 0.041). Representative data are shown in Figure 2 and Table 2.
| Lesion | TGF-LI, No. (%) | Mean Total Score ± SD | |||
|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | ||
| Non-dysplasticOLP (n = 15) | 8 (53.3) | 6 (40) | 1 (6.7) | 0 | 0.53 ± 0.64 |
| Dysplastic OLP (n = 15) | 0 | 0 | 6 (40) | 9(60) | 2.60 ± 0.51 |
| P-value | 0.041 | ||||
5. Discussion
OLP malignant transformation into OSCC has been reported (17). Among the malignancies of the oral cavity, squamous cell carcinoma (SCC) is the most frequent with a mortality rate of 50%, which despite improvement in treatment approaches has not been reduced in the last 50 years (1, 18)
The WHO recognizes OLP as a lesion with the potential for malignant transformation (PMOL) and recommends close monitoring of OLP patients. The nature of the malignant transformation in OLPs has been investigated in various studies with conflicting results, and the exact mechanism of the transformation into malignancy in these lesions is unknown. A recent hypothesis suggested that chronic stimulation of inflammatory and stromal cells leads to the generating signals that cause DNA alteration and degradation in epithelial cells and ultimately neoplastic transformation. OLP has recently been introduced as an ideal model of inflammation-induced cancers (19).
Clinical manifestations of a large number of early-stage oral cancers are premalignant lesions with the potential to develop into malignancies. Preventive therapies can therefore be employed by using biomarkers to assess the disease in its early stages (4).
TGF-β is a multifunctional cytokine that, is involved in oncogenesis in addition to its role in the immune system and inflammation, It inhibits the growth and induces apoptosis of keratinocytes in vitro, so the TGF-β1 secreted by T-cells may play a role in OLP pathogenesis. Moreover, the increased expression of TGF-β in epithelial keratinocytes may lead to the developing carcinoma in OLP lesions (20).
In this work, in addition to the expression of TGF-β in sub-epithelial lymphocytes and the significant difference between the dysplastic and non-dysplastic OLP groups, we also observed a significant overexpression of TGF-β in the dysplastic OLP epithelium compared to non-dysplastic type. This is in agreement with other studies in which the expression of TGF-β1 was implicated in dysplastic oral lesions and OSCC (21, 22).
In the present study, TGF-β was also expressed in subepithelial lymphocytes, which is consistent with Khan et al., who examined the cell-mediated immune response in OLP using IHC and ELISA. They observed the expression of TGF-β in sub-epithelial lymphocytes, which, similar to our results, imply a possible deficiency in the activity of this cytokine or related receptors. Contrary to our results, they reported the low expression of TGF-β in keratinocytes and recommended further research on the expression of this marker in OLP epithelial keratinocytes. Moreover, other studies reported that increased production of INF-γ by Th1 CD4+ T-cell in OLP lesions reduces the immunosuppressive effect of TGF-β1 (20).
Several studies have reported that, although TGF-β1 acts as a growth inhibitor in the early stages of tumorigenesis, it causes tumor growth and invasion in further stages. TGF-β is one of the main EMT-inducing and multifunctional cytokines with a major role in proliferation, differentiation, migration of cancerous cells as well as cancer progression, and metastasis (23).
The immunoreactivity of TGF-β1 in dysplastic OLP was significantly higher than that in nondysplastic OLP in our study. This is also consistent with Chen et al., who investigated the expression of TGF-β in the carcinogenesis of OLP. They showed that TGF-β-related immunoreactivity in atrophic OLP and OSCC tissue samples was higher than that in non-atrophic OLP and normal mucosal tissues. Similar to our results, they also showed the expression of TGF-β in the cytoplasm of the basal layer keratinocytes of epithelium and subepithelial lymphocytes. This indicates that atrophic OLP is more prone to malignancy than non-atrophic type (13).
Studies have also shown that TGF-β1 increases angiogenesis and the expression of matrix metalloproteinases (MMPs); the latter leads, in turn, to the activation of TGF-β1. As a result, TGF-β1 can induce its carcinogenesis through MMPs (24).
In general, inadequate immunosuppression by TGF-β-secreting, T-cells is associated with decreased TGF-β activity for reasons such as blockade of TGF-β1 secretion, dysfunctional TGF-β1 secretion, insufficient expression of TGF-β receptors, and intracellular defect in the TGF-β receptor signaling pathways which trigger hyperactive immune responses. On the other hand, high TGF-β activity, especially in the epithelium, are likely to suppress antitumor immune responses and play a role in promoting the carcinogenesis of OLP (20).
5.1. Conclusions
The results of this study indicate that TGF-β, as a marker associated with inflammatory processes in chronic inflammatory conditions, is expressed in epithelial cells and subepithelial lymphocytes of either dysplastic or non-dysplastic OLP, suggesting its possible role in the pathogenesis of this lesion. In addition to the expression of TGF-β in inflammatory cells, its increased expression in epithelial keratinocytes, as a marker involved in carcinogenesis, might play a role in the developing carcinoma in OLP lesions.
Further studies are required to investigate the function and expression of TGF-B as a therapeutic target for the management of premalignant oral lesions.