1. Background
Breast cancer is the most commonly diagnosed non-cutaneous malignancy worldwide and the leading cause of cancer-related death among females (1). Over time, significant improvements have been made in the local treatment of breast cancer with an evolution from radical mastectomy and complete axillary dissection to breast-conserving surgery and sentinel lymph node biopsy (2). Later on, adjuvant therapies such as chemotherapy and whole breast irradiation were also introduced. In an attempt to decrease regional recurrence, the inclusion of regional lymph nodes in radiotherapy (RT) portals was also examined. The benefit of this therapy for locally advanced breast tumors was shown in several clinical trials. Irradiating internal mammary lymph nodes (IMNs) showed the potential to reduce local and distant recurrence, while also improving survival in breast cancer patients with nodal involvement. Node-positive postmastectomy patients showed a significant reduction in the rate of local recurrence from approximately 30% to 10% along with a 9% improvement in the 10-year overall survival rate (3, 4). Most of the trials on regional lymphatic irradiation necessitated IMN inclusion in their protocol (5, 6). However, this was associated with a concern about the development of complications in organs close to the irradiation field. These events included but were not limited to pericardial disease, cardiomyopathy, coronary artery disease (CAD), valvular disease, pulmonary fibrosis, radiation pneumonitis, skin dermatitis, contralateral breast cancer, and esophagitis (7). Due to this reason, so far, no definite consensus has been made on the inclusion of IMNs in RT treatment portals (8, 9).
To date, various techniques have been postulated to irradiate the IMNs while minimizing the risk of toxicity in adjacent organs. In the photon/electron (P/E) technique, the medial tangent portal is matched to a mixed photon-electron anterior field, which weighs in favor of the electron (10). In the partially wide tangent (PWT) technique, the conventional medial tangential field is modified to cover superior parts of IMN (first 3 intercostal spaces) and below the top of the 4th rib head, the field is blocked to a width similar to that of standard tangents (11).
Previous studies comparing the mentioned techniques with standard and wide tangents showed that standard tangents provided significantly lower mean doses to the IMN than other methods (12). The P/E technique was useful in minimizing lung irradiation because of lower electron penetrance, but it provided an inadequate dose to the IMN due to its inherent cold spots (13). On the other hand, the PWT technique along with computed tomography (CT) planning provided sufficient coverage of the target volume (14). However, the latter technique was shown to result in dose inhomogeneity in the irradiated volume and also caused cardiopulmonary toxicity (15).
Discrepancy still exists between the findings of different studies. With the advent of potent systemic therapies and less distant recurrences, locoregional control has become more important in defining breast cancer survival. Thus, the importance of designing an optimal and precise radiotherapy technique has been emphasized in recent years. The investigation of many treatment planning techniques is required, both conventional and modern before a solution is reached.
2. Objectives
We discuss the dosimetric differences between the PWT and P/E mix techniques in terms of target coverage, dose homogeneity, and also critical organ sparing to provide additional evidence for future decisions.
3. Methods
3.1. Patient Selection
This was a simulation study. Data were obtained from CT images of consecutive patients with left-sided breast cancer referred to our clinic for either post-mastectomy or post-lumpectomy radiotherapy. The inclusion criteria were being older than 18 years, having a confirmed diagnosis of cancer in the left breast (stage pT1-3, N1-3, M0), and having undergone prior surgical procedures (irrespective of the type of surgery) with negative margins at the time of the simulation. After the eligible subjects were identified, the aims and method of the study were thoroughly explained. Informed written consent was obtained from each participant to gain permission for using their CT simulation images. The patients were reassured that entering this study would not affect the standard treatment plan that they were initially assigned by their physicians. This study was performed following the Declaration of Helsinki and its later amendments and was approved by the Ethics Committee of Tehran University of Medical Sciences.
3.2. Image Acquisition
A simulation CT scan was performed at a 5mm slice thickness from the 6th cervical vertebra to the middle of the abdomen at quiet respiration. Tattoos were used to address and locate the isocenter in the treatment position. Patients were positioned supine on the breast board on the CT couch with their left arm elevated above their head at an angle of 90 degrees or more (based on patients' compliance), the right-hand parallel to the body’s alignment, and their heads turned to the contralateral side. This positioning was maintained for all treatment sessions. The breast board angle was set to compensate for the chest slope. Thus, the posterior border of the tangent was made more parallel to the chest. We did not utilize any motion management or respiratory movement control techniques like breath-hold or respiratory gating.
3.3. Target Delineation
Clinical target volume (CTV) included both the left breast and IMN based on the radiation therapy oncology group (RTOG) consensus and with aid of a clinical reference point at the time of simulation (16). CTV of the left breast for patients with breast-conserving surgery was defined as the visible parenchyma of the left breast on the CT stimulation system and it was limited to the posterior aspect of the ribs (pleural surface) posteriorly and the skin surface anteriorly. In mastectomy patients, the volume included the residual tissue of the chest wall between the skin and pleura. The lateral margin for both groups was the typical mid-axillary line excluding the latissimus dorsi muscle. The inferior margin was considered as the level of loss of CT apparent breast and contralateral breast for lumpectomy and mastectomy patients, respectively. The superior margin was below the clavicular head.
CTV of the IMN was delineated by adding a 5mm anatomical margin around the internal mammary vessels. The internal mammary chain included the region between the superior aspect of the medial 1st rib and the cranial aspect of the 4th rib head. Planning target volume (PTV) was generated by adding a 5mm posterior, anterior, and medial geographical margin to the CTV. Also, the lateral, inferior, and superior PTV margins were extended by 1 cm.
3.4. Delineating Organs at Risk
The heart was contoured from the apex to the base of the right ventricle, the right atrium, and the auricle excluding the pulmonary trunk, root of the ascending aorta, and the superior vena cava. Both lungs were contoured bilaterally excluding the main bronchi and carina. The contralateral breast was also contoured based on visible parenchymal tissue. The esophageal Dmax (maximal dose received by the esophagus) was also measured. The treatment planning software used in this study was PCRT 3D (TRF group, Zaragoza, Spain).
3.5. Portal Design
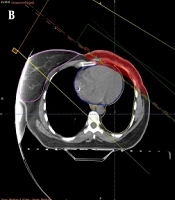
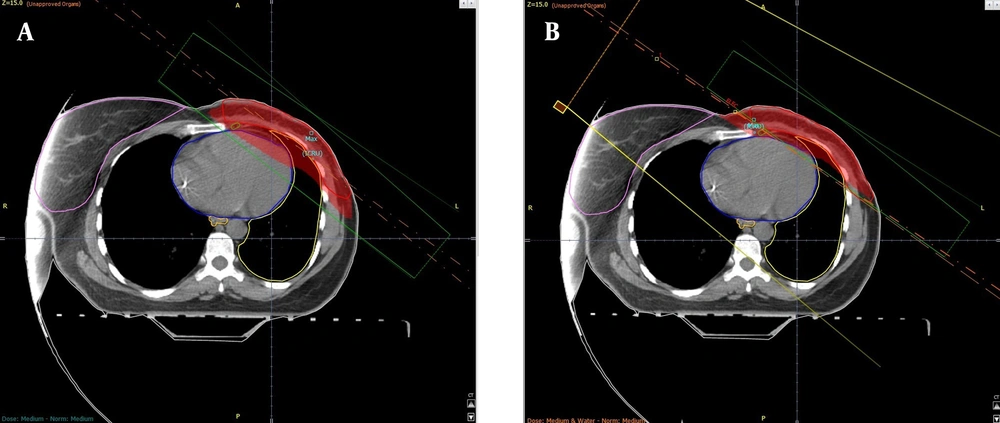
In the first method known as the partially wide tangent (PWT), two-photon fields were used simultaneously. Considering the deep penetration of photon beams, the two fields were set 180 degrees apart from each other to irradiate the tumor tissue of the breast from both sides. Standard tangents were modified manually to fully cover the contoured IMN volume. The remained unwanted areas were shielded (Figure 1A). For the second method, the P/E mix, a direct portal consisting of an electron and low-energy photons (approximately with a ratio of 70%: 30%) was used medial to the medial tangent. This portal was angeled to match the medial tangent (Figure 1B).
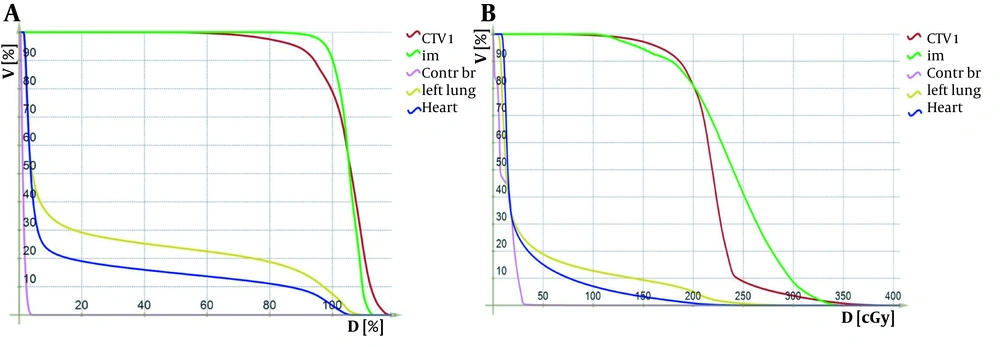
Dose-volume histograms (DVHs) were generated for the left breast, right breast, IMNs, lungs, heart, and esophagus. The maximal, minimal, and mean doses with standard deviations were calculated for the PTV. Also, the mean dose received by the IMN was calculated. Considering 2 Gy per fraction, the volumes of the left breast receiving 95% (1.9 Gy), 105% (2.1 Gy), 107% (2.14 Gy), and 108% (2.16 Gy) of the dose were obtained from the DVH curves. Also, the volume of the lung receiving 20 Gy (V20) and the heart V30 and esophageal maximal dose were calculated (Figure 2A and B). All the patients were planned, using “RT-dose planning software”, with 6 MeV photon beams.
Radiotherapy treatment parameters include field location, beam energy, administered dose for the target volume or the random dose in Dmax or air, number of fields, size of the field, total administered dose, total dose received by the tumor, and the dose administered in each session and also interpersonal differences such as anatomical variations, contour irregularities, and heterogeneity of internal tissues were taken into account in all analyses.
3.6. Statistical Analysis and Sample Size
The sample size was estimated based on a two-mean comparison. According to previous similar studies comparing these two techniques, the desired number of participants was 18 in each group (17). Gathered data were entered into SPSS version 22 (IBM Corp, Chicago, IL, USA) for analysis. Results were presented as mean percentage (standard deviation). Two-sided student t test and ANOVA were performed to identify any correlation between quantitative factors. A P-value of less than 0.05 was considered statistically significant.
4. Results
4.1. Left Breast Planning Target Volume and Internal Mammary Lymph Nodes Coverage
Although the maximal and mean doses for the left breast were greater with the P/E mix technique than with the PWT technique (P < 0.001), there was no significant difference regarding the minimum dose received by the left breast (P = 0.99). Among all patients, the volume of the left breast that received 105%, 107%, and 108%of the dose was found to be significantly higher in the P/E mix technique compared to the PWT method (P < 0.001). This means that only at the 95% dose level, the two mentioned RT methods did not differ in terms of the percentage volume of the left breast irradiated (P = 0.09). Across the total population, the P/E mix plan provided a significantly higher coverage of the IMN compared to the PWT technique (P < 0.001) (Table 1).
| Variables | Plan | Type of Surgery | P Value b | |
|---|---|---|---|---|
| MRM | BCS | |||
| CTV V95% (1.9 Gy/fraction) | PWT | 86.30 ± 4.77 | 90.44 ± 3.13 | 0.009 |
| P/EMix | 88.30 ± 7.43 | 94.49 ± 3.55 | 0.019 | |
| P value c | 0.087 | 0.319 | 0.015 | < 0.001 |
| CTV V105% (2.1 Gy/fraction) | PWT | 31.03 ± 14.83 | 32.15 ± 9.66 | 0.806 |
| P/EMix | 54.62 ± 22.09 | 72.15 ± 19.20 | 0.036 | |
| P value c | < 0.001 | < 0.001 | < 0.001 | 0.170 |
| CTV V107% (2.14 Gy/fraction) | PWT | 19.20 ± 12.40 | 18.17 ± 7.17 | 0.775 |
| P/EMix | 44.99 ± 23.12 | 61.14 ± 23.44 | 0.091 | |
| P value c | < 0.001 | < 0.001 | < 0.001 | 0.299 |
| CTV V121% (2.42 Gy/fraction) | PWT | 0.00 ± 0.00 | 0.00 ± 0.00 | - |
| P/EMix | 6.83 ± 7.21 | 11.15 ± 12.36 | 0.327 | |
| P value c | < 0.001 | < 0.001 | 0.011 | 0.311 |
| PTV D min (cGy) | PWT | 26.85 ± 20.71 | 18.13 ± 11.60 | 0.151 |
| P/EMix | 26.60 ± 22.06 | 18.77 ± 9.46 | 0.295 | |
| P value c | 0.992 | 0.971 | 0.894 | 0.104 |
| PTV D max (cGy) | PWT | 231.68 ± 5.34 | 231.05 ± 8.67 | 0.837 |
| P/EMix | 348.03 ± 59.79 | 403.49 ± 66.58 | 0.041 | |
| P value c | < 0.001 | < 0.001 | < 0.001 | 0.229 |
| Mean dose PTV | PWT | 200.22 ± 7.76 | 202.89 ± 1.86 | 0.159 |
| P/EMix | 210.82 ± 12.09 | 219.52 ± 12.88 | 0.093 | |
| P value c | < 0.001 | 0.002 | 0.001 | 0.094 |
| Mean dose IMN | PWT | 201.31 ± 9.44 | 198.10 ± 8.99 | 0.376 |
| P/EMix | 236.77 ± 34.71 | 267.07 ± 60.77 | 0.092 | |
| P value c | < 0.001 | < 0.001 | 0.002 | 0.226 |
Abbreviations: MRM, modified radical mastectomy; BCS, breast conserving surgery.
a Values are expressed as mean ± SD.
b P-value calculated from the comparison of MRM and BCS groups within each of the two radiotherapy plans (PWT and P/E mix).
c P-value calculated from the comparison of PWT and P/E mix plans within each of the two groups of type of surgery.
4.2. Heart, Lungs, Esophagus, and Contralateral Breast Volume
The toxicity of these techniques was analyzed by comparing the volume of adjacent critical structures irradiated with a specific dose. Table 2 shows the comparison of the percentage volume of the heart, lungs, and contralateral breast and the maximum radiation dose to the esophagus concerning patients’ type of surgery. As demonstrated, the PWT technique irradiated a larger volume of the heart at the 30 Gy dose level compared to the P/E mix technique (P = 0.021). This was also true about the lungs with the V20Gy being significantly higher in patients treated with the PWT technique (P = 0.003). In contrast, the volume of the contralateral breast irradiated and the maximum dose delivered to the esophagus was significantly higher with the P/E technique (P < 0.001).
4.3. Mastectomy Versus Breast Conservation Surgery
Among a total of 30 patients, 10 (33.3%) had undergone breast conserving surgery (BCS) and 20 (66.7%) had modified radical mastectomy (MRM). The mean depth of IMN (from overlying skin) was significantly higher in the BCS group in comparison to patients in the MRM group (44.6 ± 8.8 mm vs. 31.7 ± 7.6 mm, P = 0.001). Regardless of the radiotherapy treatment plan, the volume of the left breast that received 95% of the administered dose (1.9 Gy per fraction) was found to be markedly higher in the BCS group compared to the MRM group (P < 0.001), but there was no statistically significant difference across these subgroups regarding the volume of the left breast that received 105%, 107%, and 108% of the administered dose (P = 0.17, 0.30, 0.36, respectively). Also, no significant difference was observed in the maximum, minimum, and mean dose of radiation received by the left breast and the mean dose received by IMN based on the type of surgery (P = 0.23, 0.10, 0.09, 0.23, respectively).
Table 1 shows the results of dosimetry data for both techniques based on patients’ surgical procedures. Subgroup analysis showed that in patients who had undergone MRM, no significant difference existed between the PWT and P/E mix method (P = 0.319) concerning left breast V95%, but among patients with BCS, this figure was significantly higher with the P/E mix treatment plan (P = 0.015). Also, the results indicated that the P/E mix plan would raise the mean dose delivered to the IMN by 35.5 cGy/fraction in MRM patients and 68 cGy/fraction in BCS patients compared to the PWT technique (P < 0.001, P = 0.002, respectively).
The left breast V95% and V105% were found to be the greatest among BCS patients with the P/E treatment plan (94.5%) and the least in MRM patients receiving the PWT plan (86.3%). As for the left breast V107% and V108%, the highest value was similarly observed in BCS patients with the P/E mix plan, but the least was related to BCS patients irradiated with the PWT technique.
When comparing radiation toxicity to adjacent structures, in patients who had undergone a mastectomy, the V20Gy of lungs was 6.8% higher with the PWT technique than the P/E plan (P = 0.005); however, for patients with BCS, no significant difference existed (P = 0.109). In addition, a non-significant increase of 4.4% and 2.7% in the volume of the heart irradiated with the PWT method was observed among BCS and MRM patients, respectively (P = 0.06 and 0.08). As for toxicity to the right breast and esophagus, the P/E technique contributed to a statistically significant larger irradiated volume across both surgery subgroups. The esophageal maximal dose was highest in BCS patients treated with the P/E method (2.01Gy) and least in MRM patients receiving the PWT treatment plan (0.7Gy) (Table 2).
| Variables | Plan | Type of Surgery | P Value b | |
|---|---|---|---|---|
| MRM | BCS | |||
| Heart V30Gy | PWT | 7.03 ± 4.15 | 13.60 ± 4.02 | 0.001 |
| P/EMix | 4.36 ± 4.39 | 9.24 ± 6.03 | 0.039 | |
| P value c | 0.021 | 0.056 | 0.076 | < 0.001 |
| Lung V20Gy | PWT | 27.40 ± 6.09 | 34.67 ± 8.48 | 0.030 |
| P/EMix | 20.56 ± 8.25 | 27.25 ± 10.99 | 0.111 | |
| P value c | 0.003 | 0.005 | 0.109 | 0.012 |
| Esophageal D max (Gy) | PWT | 7.04 ± 1.06 | 10.17 ± 2.52 | 0.003 |
| P/EMix | 15.97 ± 7.90 | 20.06 ± 8.47 | 0.220 | |
| P value c | < 0.001 | < 0.001 | 0.002 | 0.095 |
| Contralateral breast V5% | PWT | 1.21 ± 3.75 | 3.84 ± 4.20 | 0.114 |
| P/EMix | 20.74 ± 20.72 | 41.51 ± 16.96 | 0.008 | |
| P value c | < 0.001 | < 0.001 | < 0.001 | 0.033 |
Abbreviations: MRM, modified radical mastectomy; BCS, breast conserving surgery.
a Values are expressed as mean ± SD.
b P-value calculated from the comparison of MRM and BCS groups within each of the two radiotherapy plans (PWT and P/E mix plans).
c P-value calculated from the comparison of PWT and P/E mix plans within each of the two groups of type of surgery.
5. Discussion
Irradiation of the whole breast after surgery is the standard of care in many patients with breast cancer or pre-invasive lesions. The inclusion of IMNs in the irradiation field gained attention many years ago to control common occult lymph node metastases. Nonetheless, initial results were unsatisfactory and non-cancer-related mortality and morbidity mainly owing to cardiac and lung complications were a valid concern (18-20). Advances in radiotherapy delivery methods, however, resulted in enhanced treatment outcomes. In 2012, after an average follow-up of 6.2 years, Olson et al. reported that IMN radiation was not associated with significantly improved survival in all patients; however, they suggested that its application in individuals with low-burden lymph node involvement (N1) could improve patients’ outcome (21). Later in 2015, a study on approximately 4 000 patients with early-stage breast cancer showed significantly improved disease-free survival, less distant metastasis, and marginal improvement in overall survival. Moreover, while a significantly reduced breast cancer-related mortality was achieved, no treatment-related mortality occurred (6). In the same year, the results of another large study known as the MA.20 trial was published, which indicated that compared to whole breast irradiation alone, patients treated with comprehensive regional irradiation plus whole-breast irradiation had a reduced risk of breast cancer recurrence, both in the regional nodes and at distant sites (5).
A persistent dilemma exists regarding the best radiotherapy technique that provides IMN coverage while contributing to fewer adverse events. In the present study, we found a higher mean dose delivered to the IMN by the P/E technique compared to the PWT method. This was in contrast with the results published by Severin et al., but consistent with the findings of a more recent study by Dumane et al. in 2014 (17, 22). In the latter study, the V95% for the target, which included the chest wall and the nodes, was > 95% for both the PWT and photon/electron techniques (22). Nonetheless, the highest V95% observed in our study was 94.5% in BCS patients treated with the P/E plan.
In line with previous studies, we found that the formation of hotspots was significantly more prevalent among patients treated with the P/E mix plan (17, 23). When comparing results across mastectomy and lumpectomy patients, our study demonstrated that the P/E technique retained many of its advantages of target coverage and toxicity regardless of the type of surgery. This finding was similar to the results of the study by Severin et al. The authors of the mentioned study, however, reported a significant difference regarding the mean dose of the IMN in patients treated with the PWT plan based on their primary surgical procedure (17). We did not observe such a difference in our study.
Previous studies have been inconclusive regarding the superiority of a specific technique in causing less radiation-induced toxicity. Some studies have shown that the PWT technique leads to an increased depth of normal tissue exposed to radiation and, thus, have proposed that using a combination of photons and electrons could significantly decrease the amount of lung and heart exposure to high-dose radiation (17, 22, 24). On the other side, several studies indicated that cardiac substructures receive more radiation exposure after radiotherapy with a P/E beam and the least exposure with PWT (23, 25). In the present study, we found a significantly higher volume of the heart and lungs to be irradiated with the PWT plan compared to the P/E technique. Also, in both techniques, the extent of heart and lung exposure within BCS patients was greater than in MRM patients, which was in contrast to the results of a previous study (17).
Previous studies have shown that significant radiological and symptomatic radiation pneumonitis does not usually occur unless the V20Gy of the lung is above 30% (26, 27). In the present study, only MRM patients irradiated with the PWT technique reached this threshold. As for cardiac toxicity, long-term follow-up of patients in randomized clinical trials revealed that radiation exposure of the heart during breast cancer radiotherapy increases the subsequent risk of heart disease (28). Marks et al. proposed that radiation therapy causes volume-dependent cardiac perfusion defects in approximately 40% of patients within 2 years of RT. They showed an incidence of < 20% for fields involving < 5% of the LV versus > 50% when > 5% of the LV was irradiated (29). In another study, Wei et al. reported the V30 of the heart as a predictor for pericardial effusion with the risk of effusion being 13% and 73% if V30 was below or above 46%, respectively (30). In this study, the largest mean volume of the heart irradiated was 13.6% with the PWT and 9.2% with the P/E technique, meaning a low risk of effusion for both techniques, but a relatively high possibility of perfusion defects. As such, reducing post-radiation heart damage remains an important goal to be achieved.
Another organ that is at risk of unintended radiation and, thus, requires protection is the contralateral breast. A recent study investigating internal mammary nodal radiation for breast cancer showed an increase in the overall survival of patients; however, this came at the cost of a higher incidence of contralateral breast cancer in the survivors (31). Severin et al. showed that the volume of the contralateral breast receiving 2.5 Gy (5% of the prescribed dose) was 24.5% with PWT and 4% with P/E (17). This was in contrast with the results of our study, which indicated a noticeably greater volume of the right breast being irradiated with the P/E plan compared to the PWT technique. Dumane et al. reported differences in doses to the contralateral breast to be insignificant amongst all 3D techniques (22).
A study conducted in 2003 by Fiets et al. showed that including regional lymph nodes in the target volume (with a dose of 2 Gy per fraction) is associated with a significantly higher risk of esophagitis or dysphagia as 53% of patients treated with locoregional radiotherapy concurrent with chemotherapy developed high-grade esophagitis or dysphagia, compared to only 12% of patients treated with local radiotherapy. They concluded that the type of primary surgical treatment was not significantly associated with any of these complications (32). This finding was consistent with the results of our study, indicating no significant difference in the maximum dose delivered to the esophagus based on patients’ type of surgery. In addition, several previous studies have indicated an increased risk of esophageal carcinoma among patients undergoing radiotherapy for breast cancer. However, due to a lack of information, the dose level, which would contribute to esophageal carcinoma, was not specified (33, 34).
As with any other study, this study was also associated with some limitations. For example, in this study, we did not investigate the effect of variables such as the depth of the ipsilateral lung, chest wall separation, and length of the lung on our outcomes. Also, the patient’s characteristics such as body size, weight, breast size, and also the stage of disease were not included in our analysis. Moreover, the outcomes of only two radiotherapy techniques were compared. Thus, we suggest a larger trial that provides dosimetric data of further advanced techniques and conducts subgroup analysis to measure the effect of the mentioned variables.
5.1. Conclusions
In summary, the P/E mix plan provided a higher target coverage of the left breast and the IMNs plus more sparing of the heart and lungs. However, these benefits came at the cost of a higher dose to the esophagus and greater contralateral breast irradiated volume compared to the PWT technique. Considering this, we recommend that when designing treatment plans, clinicians take into account on an individual basis the competing risks of each technique and whether or not to include regional lymph nodes.