1. Background
Cancer is a multistage genetic disease in which successive mutations lead to abnormal changes in the DNA of genes and directly affect the cells, their growth process, and division, which cause cancerous tumors. Genetic changes ending in cancer can be inherited from parents or be caused by damage to DNA by environmental factors, including chemical materials, sunlight radiations, and electromagnetism during life (1). Gastric cancer is one of the serious health problems worldwide and is the third most common type of cancer that causes death (2). Several major groups of genes have been involved in gastric cancer, including oncogenes, tumor suppressor genes, and genes involved in DNA repair.
Given the high number of people with various types of cancer, the effectiveness factors have been examined in several studies, one of which is electric and magnetic fields (EMFs). From the very starting reports, the relationship between cancer incidence and exposure to very low-frequency electromagnetic fields (ELF-EMFs) has been identified as a possible carcinogen by the International Agency for Research on Cancer (IARC) (3). Thus, many research groups have focused on various biological effects of ELF-EMF like the changes in fetal development stages, genotoxic effects, and alterations in genes expression. Nonetheless, some reports have suggested that EMFs do not affect biologically (4-8). By the increasing use of electrical appliances in the 20th century, electromagnetic radiation, especially low-frequency radiation, started to spread more in the environment. These fields have various biological effects depending on their density, frequency level, and radiation duration (9).
The interaction between magnetic fields and biological systems may be positive or negative depending on the prevailing condition of the encounter. Magnetic fields change function and metabolism by disrupting cell structure and altering enzymes (10).
The first report on the relationship between electromagnetic fields and cancer was found in the study of cancer and mortality in children exposed to high levels of electromagnetic fields. EMFs could increase leukemia or lymphoma in children exposed to 2 to 3 times electromagnetic fields compared to those exposed to fewer amounts (11). Sengupta and Balla examined the use of magnetic fields for cancer treatment and noted the general effects of magnetic fields on the body, including increased blood circulation and stimulation of the body's metabolism. They found that the use of magnetic fields produced free radicals and induced apoptosis in cancer cells (12).
Suppressors of cytokine signaling (SOCS) family proteins form part of a classical negative feedback system regulating cytokine signal transduction. SOCS3 is a tumor suppressor identified as upstream of JAK/STAT3 (Janus kinase/signal transducers and activators of transcription 3) signaling by specific kinase inhibition. Low expression of SOCS1 (suppressor of cytokine signaling 1) and SOCS3 was considered a poor prognostic indicator for gastric cancer patients. SOCS3 was also classified as a potential indicator for predicting the lymph node metastasis from gastric cancer (13-15). In the study of colorectal cancer, the expression of this gene has been greatly reduced, and it can be used as a target for gene therapy (16).
Cathepsin V (CTSV/CTSL2) gene, a lysosomal cysteine protease, is a member of the cathepsin family, which is linked to cancer invasion and metastasis. It is responsible for destroying the extracellular matrix. Studies have shown that this gene can have a significant role in corneal physiology. This gene is expressed in colorectal and breast carcinomas, but not in the colon, mammary glands, and natural tissues, suggesting a possible role in tumor processes. It is associated with the poor prognosis of breast cancer (17, 18).
2. Objectives
Due to the role of CTSL2 and SOCS3 in cancer progression, the objective of the present study was to evaluate the impact of ELF-EMFs with magnetic flux densities of 0.2 and 2 mT continuously and discontinuously (1.5 h on/ 1.5 h off) for 18 hours on the expression changes of these two genes in a human gastric cancer cell line (AGS).
3. Methods
3.1. Cell Culture
In this study, the gastric cancer cell line (AGS) was prepared from the Iran Genetics Resource Center and was cultured in Hams12 culture medium, with FBS 10% and Penicillin-Streptomycin 0.01% (all from Gibco) in a filter flask (T25). The cells were incubated in a 5% Co2 incubator at 37°C and a suitable humidity. The cells were washed daily with 3 - 2 mL PBS (Peripheral Blood Smears) and the culture medium was changed. The cell passage was done after covering 85% of the culture medium volume of cells. To detach the cells from the bottom of the flask and transfer them to the new culture medium, 25% trypsin EDTA (Ethylenediamine tetraacetic acid) was used. The cells with the new culture medium were placed inside the flasks in the incubator, and finally the cells were divided into control groups and electromagnetic field exposed groups.
3.2. Electromagnetic Field Induction System
The exposure mechanism consists of a solenoid cylinder with a diameter of 12 cm, a height of 30 cm, and 1200 revolutions of copper wire with a diameter of 1 mm in 4 rows (19). Electromagnetic fields were produced by alternating current (AC) power supply (model: TDGC2, 220 v, 50 - 60 Hz Delta International Electric Co, Shanghai China). The control and exposure groups were incubated under a fixed condition of temperature, humidity, and CO2. The cells were exposed to electromagnetic fields densities of 0.2 and 2 mT continuously and discontinuously (1.5 h on/ 1.5 h off) for 18 hours. The magnetic fields inside the solenoid were measured by a digital Holladay three-D sensor (Holladay, Eden Prairie, MN). It has to be noted that increases in current would increase the temperature inside the solenoid. As the cells are damaged at temperatures higher than 39°C, the temperature inside the solenoid was controlled. Therefore, in addition to set the temperature inside the incubator at 37°C, a small ventilator was placed at the bottom of the solenoid, which created a continuous flow of air inside the incubator and the solenoid. The wooden base with a height of 15 cm was designed for solenoid so that it does not have direct contact with the metal surfaces of the incubator and is fixed in the central space of the incubator.
3.3. EMF Radiation Condition
AGS cells were exposed to the magnetic fields of of 0.2 and 2 mT continuously and discontinuously. The cells were exposed to the electromagnetic field for 18 hours so the continuous group was exposed nonstop for 18 hours and the discontinuous group was exposed to the electromagnetic field in the form of 1.5 h intervals for a total of 12 intervals. There was a control (Sham) sample for all experiments. The purpose of selecting the discontinuous exposure is that people are exposed to magnetic fields intermittently in their daily lives, because of using appliancethat produce weak electromagnetic fields in homes, workplaces, medical centers, or even the electronic tools that they carry.
3.4. Evaluation of the Cell Viability
MTT (3-(4, 5-Dimethylthiazol-2-yl)) assay method was used to assay the effect of an electromagnetic field with the desired intensities on the growth and proliferation of cancer cells. This method is a competitive mitochondrial metabolic test based on the breakdown of tetrazolium salts by succinate dehydrogenase. After passaging, a cell suspension was provided in the culture medium and cultured in a 96-well plate. The cells were incubated for 24 hours to stick to their substrate. In this test, 30 wells of the plate were considered for each intensity of the electromagnetic field. MTT solution was added to them. After the addition of the succinate dehydrogenase solution, which is one of the mitochondrial respiratory enzymes, it is redacted. Reduction and decomposition of this chain produce purple-blue crystals. The color and the number of metabolically active cells or living cells are directly related to each other. Finally, the optical absorption of each cell was measured by the spectrophotometer at the wavelength 570 nm and the average of the numbers belonging to each group was used.
3.5. RNA Extraction and Evaluation
RNA extraction (ribonucleic acid) from cells was done using the TRizol (total RNA isolation) kit according to the instructions. Quantitative and qualitative methods were used to evaluate the quality of extracting RNA, so that optical absorption, using a nanoparticle was measured at a 260/280 absorbance ratio for quantitative analyses. Optical absorption of all the samples was in the range of 1.8 - 2, which is an acceptable range. Moreover, 1% gel electrophoresis was used for qualitative examination and the extracted RNA was suitable given the band seen at 18s (Svedberg) and 28s points.
3.6. CDNA Synthesis and Evaluation
Reverse transcription and the fabrication of complementary DNA (cDNA) out of the extracted RNA were performed using a Takara kit according to the kit protocol. The PCR reaction was performed to evaluate synthesized cDNAs using specific primers, and the product was loaded onto a 2% gel. According to molecular mass, a specific band was shown at the points which confirm the specificity of the primers.
In order to find the sequence of genes, the University of California Santa Cruz (UCSC) site was used. The design of the specific primers was done by Primer3 and Oligo Analyzer software, and then the determination of primer specificity in the National Center for Biotechnology Information (NCBI) site, Primer Blast was controlled. In this study, the β-actin gene was considered as a housekeeping gene. The sequence of all primers is shown in Table 1.
| Genes | Primer Type | Sequence |
|---|---|---|
| CTSL2 | Forward | 5'- CAGTGGAAGGCAACACACAG -3' |
| Reverse | 5'- CCACAGATTTGGGAAGATCAA -3' | |
| SOCS3 | Forward | 5'- CGGAGACTTCGATTCGGGAC -3' |
| Reverse | 5'- GGTACTCGCTCTTGGAGCTG -3' | |
| β-actin | Forward | 5'- GATCAAGATCATTGCTCCTCCTG -3' |
| Reverse | 5'- CTAGAAGCATTTGCGGTGGAC -3' |
Designed Primers for Real-time RT-PCR
3.7. Real-time PCR
Real-time PCR reaction was done using Master Mix PCR kit, containing SYBR Green fluorescent dye. This marker is always bound to the minor groove of the DNA double helix placed in the groove between the 2 strands of DNA and emits light. When DNA increases, the fluorescent light increases as well. It has to be noted that to prevent pipetting hand errors for each gene, a master containing cyber green, primer, and water was prepared and the samples were tested as pairs to ensure the test results. Reactions are generally run for 40 cycles and there are 4 major temperature steps in the reaction, as follows:
First step Initialization: Denaturation of the two strands of DNA pattern. This step is required for DNA polymerases which should be thermally activated by hot-start PCR. It was done in 1 minute at 95°C. The second stage is denaturation of the double-stranded DNA template by breaking the hydrogen bonds at 95°C for 15 seconds, then annealing for 40 seconds at 60°C. In the last step, extension, the temperature rises from 66 to 92°C. The melt and amplification curves were plotted using the device and analyzed to examine the rate of changes in gene expression for the studied groups. The relative expression of the genes was calculated using 2−∆∆ct method based on the values of the cycle threshold (CT).
3.8. Data Analysis
In this study, SPSS statistical software, version 23 was used to perform all statistical analyses. Data were expressed as Mean ± SD. A P-value < 0.05 was considered statistically significant. A comparison of data among different magnetic field densities and exposure patterns was perfomed with the non-parametric Kruskal–Wallis test for numerous independent samples. Comparison of data between control and exposed samples was examined using the Post Host Tests.
4. Results
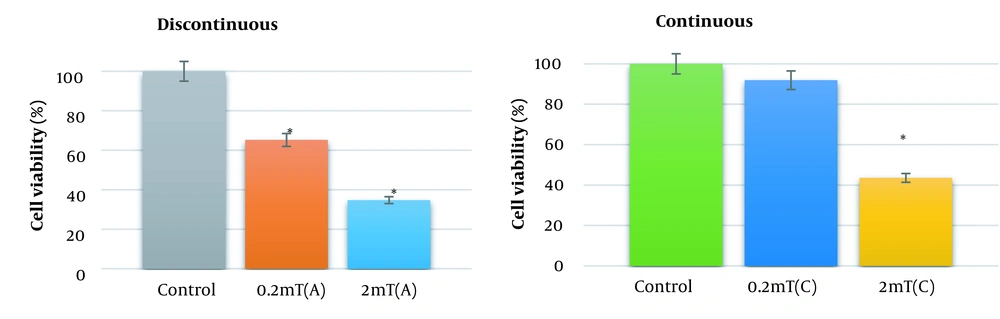
AGS cells viability was evaluated under the exposure to the electromagnetic flux density of 0.2 and 2 mT for 18 h continuously and discontinuously by MTT assay. Figure 1 reveals the changes in AGS cell viability following the exposure to ELF-EMFs. Continuous exposure to the magnetic field of 0.2 and 2 mT decreases the cell viability to 85% and 41%, respectively (P < 0.01). The viability of AGS cells was decreased to 62% and 37% after discontinuous exposure to 0.2 and 2 mT, respectively (P < 0.01).
Cell viability of AGS cells exposing to ELF-EMS with magnetic flux density of 0.2 and 2 mT. AGS cells were exposed to continuous and discontinuous magnetic flux densities of 0.2 and 2 mT for 18 h. Control samples were cultured under the same condition without the exposure to the electromagnetic field. Data are represented as the mean ± SD. C: Continuous, A: Discontinuous. *P-value < 0.05, **P-value < 0.01.
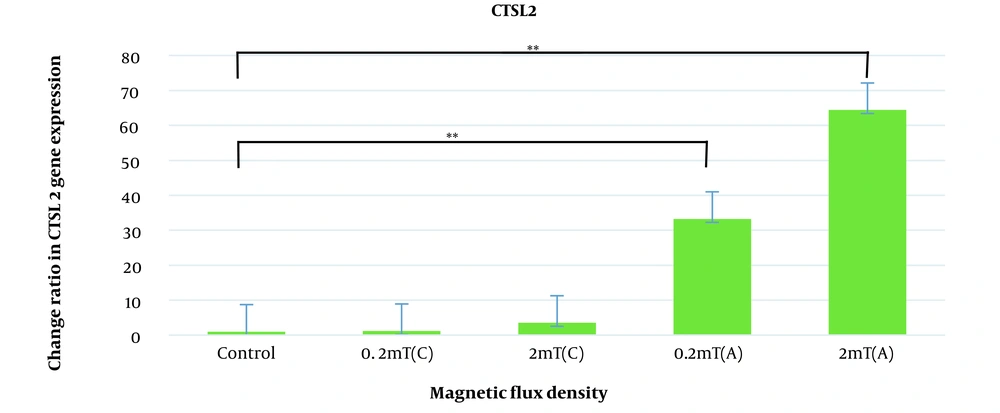
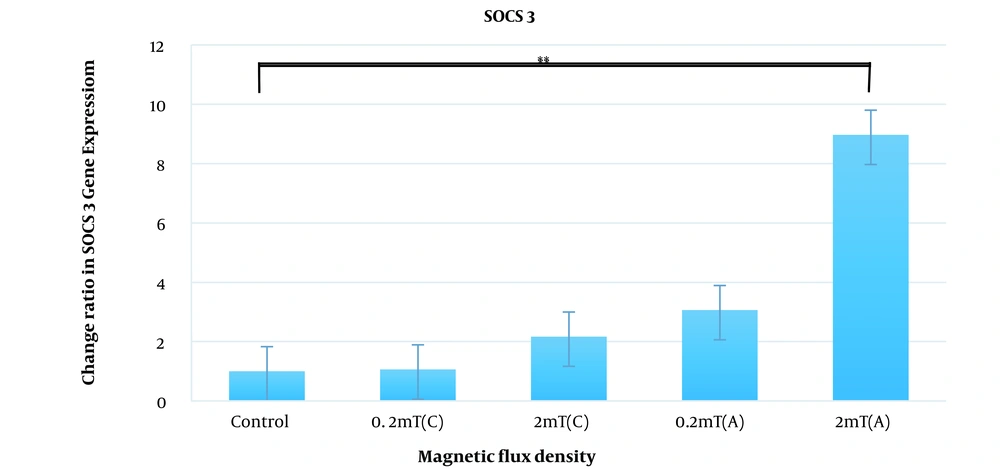
Relative gene expression under the exposure of EMFs was determined by quantitative real-time PCR compared to the control group. According to Figure 2, expression levels of CTSL2 were increased under exposure to electromagnetic fields and this increase was significant when discontinuous exposure was applied (33.26 ± 7.4 fold change for 0.2mT and 64.4 ± 7.7 for 2 mT, P- value < 0.001). SOCS3 is also up-regulated under the exposure of magnetic flux density of 0.2 and 2 mT continuously (1.06 ± 0.45 and 2. 7 ± 0.50 respectively) and discontinuously (3.06 ± 0.87 and 8.97 ± 0.95 respectively) but this up-regulation was significant at only discontinuous exposure of 2 mT magnetic flux density (P-value < 0.05) (Figure 3).
Expression changes of CTSL2 in AGS cell line under the continuous and discontinuous exposure to ELF-EMF with magnetic flux density of 0.2 and 2 mT. AGS cells were exposed to continuous and discontinuous magnetic flux densities of 0.2 and 2 mT for 18 h. Control samples were cultured under the same condition without exposure to the electromagnetic field. Data are represented as the mean ± SD. C: Continuous, A: Discontinuous. *P-value < 0.05, **P-value < 0.01.
Expression alternations of SOCS3 in AGS cell line under the continuous and discontinuous exposure to ELF-EMF with magnetic fields of 0.2 and 2 mT. AGS cells were exposed to continuous and discontinuous magnetic flux densities of 0.2 and 2 mT for 18 h. Control samples were cultured under the same condition without exposure to the electromagnetic field. Data are represented as the mean ± SD. C: Continuous, A: Discontinuous. *P-value < 0.05, **P-value < 0.01.
5. Discussion
Considering the increase in the utilization of electromagnetic generators and the uncertain mechanism of their effect on the cells, this study was intended to investigate the effect of weak (0.2 mT) and medium (2 mT) magnetic flux density on the relative expression of CTSL2 and SOCS3 genes in AGS cell line. The results indicated that a weak magnetic flux density of 0.2 mT continuously and discontinuously for 18 hours could increase the expression of CTSL2 up to 1.2 and 33.2 fold, respectively. Also, that could increase the expression of SOCS3 up to 1.06 fold in continuous groups and 3 fold in discontinuous groups. In addition, the moderate magnetic flux density of 2 mT under the same conditions and exposure time increased the expression of CTSL2 in continuous groups up to 5.5 fold and in discontinuous groups up to 64.4 fold as well as increased the expression of SOCS3 up 2 and 8.9 fold in continuous groups and in discontinuous groups, respectively.
The results indicated an increase in CTSL2 and SOCS3 gene expression in all experimental groups in a dose-dependent manner compared to the control group. Intergroup comparisons indicated that groups under discontinuous fields have significantly increased expression in comparison with the continuous groups. Correlation analysis was performed between the experimental and control groups. The results showed a significant relationship (P < 0.05) between 2 mT discontinuous group and control group in CTSL2 (P < 0.05) and SOCS3 (P < 0.001) genes. According to the results, the effects of fields on CTSL2 expression are much more significant than that of the SOCS3. The results were similar to the study of Mahmoudinasab et al. They conducted their study on the effects of fields 0.25 and 0.5 mT in breast cancer cell line and showed that chloramphenicol acetyltransferase (CAT), superoxide dismutase (SOD1), and superoxide dismutase 2 (SOD2) expression increased under the effect of electromagnetic fields (20). Moreover, Phillips showed that electromagnetic fields affected the expression of C-JUN, C-MYC, and C-FOS genes (21).
Sengupta and Balla examined the effects of a magnetic field in the treatment of breast cancer. The findings indicated that magnetic fields lead to increased blood circulation to tissues and stimulate body metabolism. Weak electrical current in tissues increases the potential of the cell membrane surface and leads to increased blood circulation, oxygen, nutrient supply, and body tissue repair. The magnetic field seems to be a potential approach to cancer treatment by controlling the secretion of cytokines and interleukins (12).
Electromagnetic fields have different biological impacts such as changes in temperature, ionic and molecular currents, the orientation of molecules, the lifetime of free radicals depending on the kind, function of the biological system, applied frequency, intensity, and time of exposure (19). Several studies have revealed that electromagnetic fields could changes expression of NOTCH1 gene and its regulatory circular RNA (circRNA), hsa_circ_0005986, in human gastric adenocarcinoma cell line (22).
MicroRNA (miR)-144 and miR- 375 up-regulated following exposure to electromagnetic fields (23).
These effects include DNA synthesis, RNA transcription, cell proliferation, transmitting intracellular messages, and gene transcriptions. Although the mechanism(s) underlying such effects have not been completely defined, their possible mechanisms are as follows:
(1) The exposure to different biological processes to EMFs has been shown to alter the membrane load by affecting ion channels and altering ion entry and exit. For instance, by acting on calcium channels, leading to the concentration changes of calcium ions inside the cell to change through the effect on calcium membrane channels, as well as the channels on the intracellular reserves of calcium ions (such as the endoplasmic reticulum). Calcium ions enter and exit (24). Ca2+ is one of the most widely used messengers in cell biology. Among the vital invaluable findings of the last decades was the role of Ca2+ in the regulation of cellular adaptation through its capability to control gene expression. The study established a connection between cell excitation and gene expression (25). Intracellular Ca2+ rised when Voltage-gated calcium channel were stimulated, which can act in turn to stimulate the two calcium/calmodulin-dependent nitric oxide synthesis and increase nitric oxide. It is suggested that nitric oxide may act in pathophysiological responses to EMF exposure, by acting as a precursor of peroxynitrite, producing both oxidative stress and free radical breakdown products (24). Overall, calcium ions directly affect the regulation of basic cellular processes like proliferation, protein synthesis, and differentiation. Many diseases can be occurred by changes in their concentration. For example, 70% of gene expression changes (up or down gene regulation) are observed in T lymphocytes from immune disorder patients and are caused by Ca2+ deficiency (26). Moreover, it controls the transcription pathway by affecting transcription factors and can change gene expressions (27).
(2) The other effect that EMFs is, epigenetic changes like the changes in gene methylation. DNA methylation is affected by the regulation of chromatin structural changes, the expression of genes involved in cell cycle controls, apoptosis and DNA repair, involved in cell growth, autoimmune diseases, cancer, and central nervous system disease. The cancer development and progression can be managed by epigenetic mechanisms like gene promoter methylation. Epigenetic alterations are heritable alternations take place in the structure and function of the genome without a change in DNA sequence. several studies have been conducted on the epigenetic changes of the tumor suppressor genes and the identification of methylation biomarkers in colorectal cancer (28). Studies have shown that electromagnetic fields cause changes in metabolic systems like metallization in the genome and changes in gene expression (29). It increases the expression of methyl transferases, and as a result, creates hypermethylation in DNA or histones (30).
In this study, the electromagnetic fields were continuously and discontinuously irradiated. Based on the findings of the present study and other studies, electromagnetic fields effect on the expression of the genes in a discontinuous state is much higher compared to a continuous state. Its cause can be considered as an adaptive mechanism in the cell so that when it is constantly exposed to the magnetic field, it adopts some mechanisms for less damage to the cell (31, 32).
Examining electromagnetic field effects on gene expression changes, especially in tumor cell lines, is of great importance, studies have been conducted in the field of treatment with them. The difference between our study and other ones is that it was performed on human gastric cancer lines, and the applied fields were considered similar to the electrical devices to which we are exposed. Moreover, the effect of the time factor and radiation was considered as well.
5.1. Conclusions
The analysis of the indices recorded in this study indicated that 18 hours of exposure to electromagnetic fields with low and medium intensity in AGS cell lines have some effects on the expression of SOCS3 and CTSL2 genes as well as that, increases their expression. The increase in expression is directly associated with the intensity of the field, so that the rate of changes in the medium radiation is greater than that of the weak radiation. Moreover, the rate of expression changes for all experimental groups in the alternating field state is much greater than that of the continuous mode.