1. Background
Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) account for about 80% and 20% of non-melanoma skin cancers (1). BCC is the most prevalent skin cancer that is mainly caused by ultraviolet (UV) exposure and changes in p53 and PTCH (patched gen) genes (2). The head and neck are the most common locations for BCC as they are more exposed to sunlight than other organs (1). Although BCCs slowly grow and rarely metastasize, they can become locally invasive if left untreated (2).
Cutaneous SCC (cSCC) is the second most prevalent type of malignant skin tumor. Such factors as human papillomavirus, old age, male sex, and status of host immunity system are among the causes of cSCC. However, similar to BCC, the mutagenic effects of UV exposure are the most common causes of this disease. Moreover, cSCC is highly prone to metastasis (3).
Although both tumors originate from non-differentiated keratinocytes, they show different biological and metastatic behaviors (4). In this regard, BCC rarely metastasizes, with a metastatic range of 0.0028% to 0.55%, while SCC has a high metastatic potential of 0.1% to 13.7% and can ultimately lead to mortality (5). Meanwhile, the mechanism responsible for the different growth patterns in BCC and SCC has not been fully elucidated yet (4).
The microenvironment of tumors, including a range of inflammatory cells other than tumor cells, is of paramount importance for the maintenance of normal tissue hemostasis or the promotion of tumor growth (6). These cells are lymphocytes, macrophages, neutrophils, plasma cells, mast cells, and eosinophils. The response of stroma to the tumor is due to the intensity of the inflammatory cells' infiltration around the tumor (7). In this regard, the dense infiltration of the inflammatory cells probably reflects the good defense of the host to the tumor. Recently, special attention has been given to the role of the mast cells and eosinophils in tumor biology (8).
The mast cells form a heterogeneous population of cells that are different in terms of structure, morphology, mediators, and surface receptors. These cells are derived from the multipotent hematopoietic stem cells in the bone marrow (9) and distributed throughout almost all tissues mostly close to the epithelium, fibroblasts, nerves, as well as blood and lymph vessels (6). Recent research has shown that the enhancement of mast cell density can be associated with a favorable tumor prognosis (10). However, some studies reported that the mast cells could be important in the progression of cancers by increasing angiogenesis (11).
Tumor-associated tissue eosinophil (TATE) is defined by the presence of eosinophils as part of peritumoral and intratumoral inflammatory infiltration (12). Eosinophils are granulocytic cells of the innate immune system that are differentiated from the bone marrow progenitor cells (13). Eosinophil infiltration is found in a broad spectrum of skin disorders. In general, the function of eosinophils in skin disorders is related to immunomodulation, host defense, fibrosis, and/or tumor development (14). The mechanism of eosinophils is partially identified in non-melanoma cancers (15, 16).
2. Objectives
Based on our searches, there is no evidence regarding the precise role of TATE and mast cells in tumors (7, 17). The infiltration of these cells has been associated with both favorable and unfavorable cancer prognoses (8). Given the lack of any studies comparing the role of these inflammatory cells between skin SCC and BCC, the present study was conducted to investigate the count of two inflammatory cells, namely mast cells and eosinophils in these common skin cancers.
3. Methods
3.1. Sampling
This descriptive-analytical study was conducted on the excisional biopsy samples of the head and neck BCC and cSCC, which were obtained from Khatam-Alanbia Hospital of Zahedan, Iran, from 2005 to 2015. The ethics committee of Zahedan University of Medical Sciences, Zahedan, Iran, approved this study under projects No.7563 (IR.ZAUMS.REC.1394.370) and No.1621 (IR.ZAUMS.REC.1394.247). The data extracted from the patient's medical records included age, gender, and histopathological type of lesions.
Location of tissue specimen were skin of the nose (26.3%), forehead (17.1%), scalp (15.8%), ear (13.2%), neck (11.8%), cheek (9.2%), and eyelids (6.6%). After examining hematoxylin and eosin staining slides and confirming the histopathological diagnosis of the lesions, the BCC samples were categorized according to the instructions of the World Health Organization (18). Histopathologic subtypes of the BCC samples were nodular, sclerosing, basosquamous, superficial, and adenoid patterns. In addition, the cSCC lesions were categorized as grades I, II, and III (well, moderate, and poor). In the well-differentiated type of tumoral cells, there are hyperchromatic nuclei and abundant cytoplasm. A lot of Keratin pearl formation is seen. Intercellular bridges are often visible. In poorly-differentiated tumors, the cells are greatly enlarged. Nuclear pleomorphism and a high degree of atypia and numerous mitoses are also seen. Keratin production is significantly reduced. In the moderately differentiated subtype, intermediate features of well-differentiated and poorly-differentiated tumors are seen (19).
3.2. Histochemistry Staining
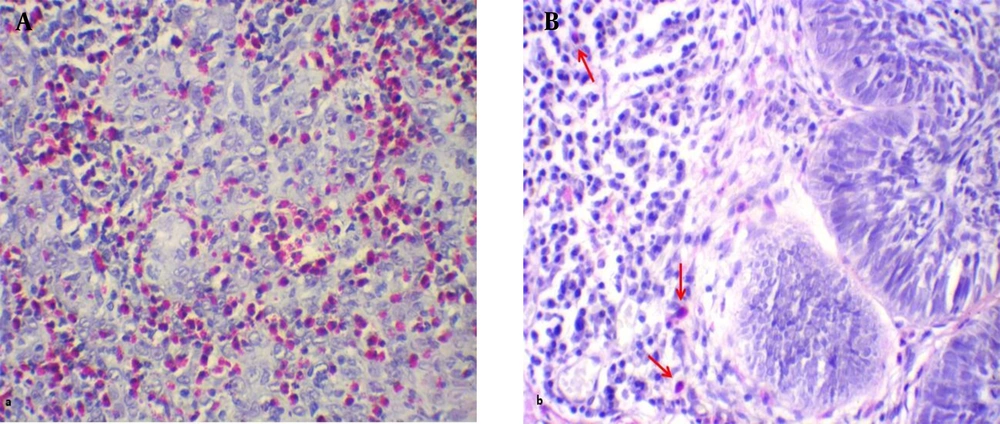
The samples with an extensive amount of necrosis and/or the paraffin blocks lacking adequate tissue were excluded from the study. Two sections of each paraffin block with 4 Micron thickness were prepared and stained with histochemical Sirius red and Toluidine blue for tissue eosinophil and tissue mast cell counts, respectively (8, 20). To this end, for Sirius red staining, the sections were placed in Harris hematoxylin for 2 min and, then, rinsed with running tap water, followed by a rinse with 100% ethanol. In the next stage, they were immersed in an alkaline Sirius red solution (pH 8 - 9) and rinsed with running tap water.
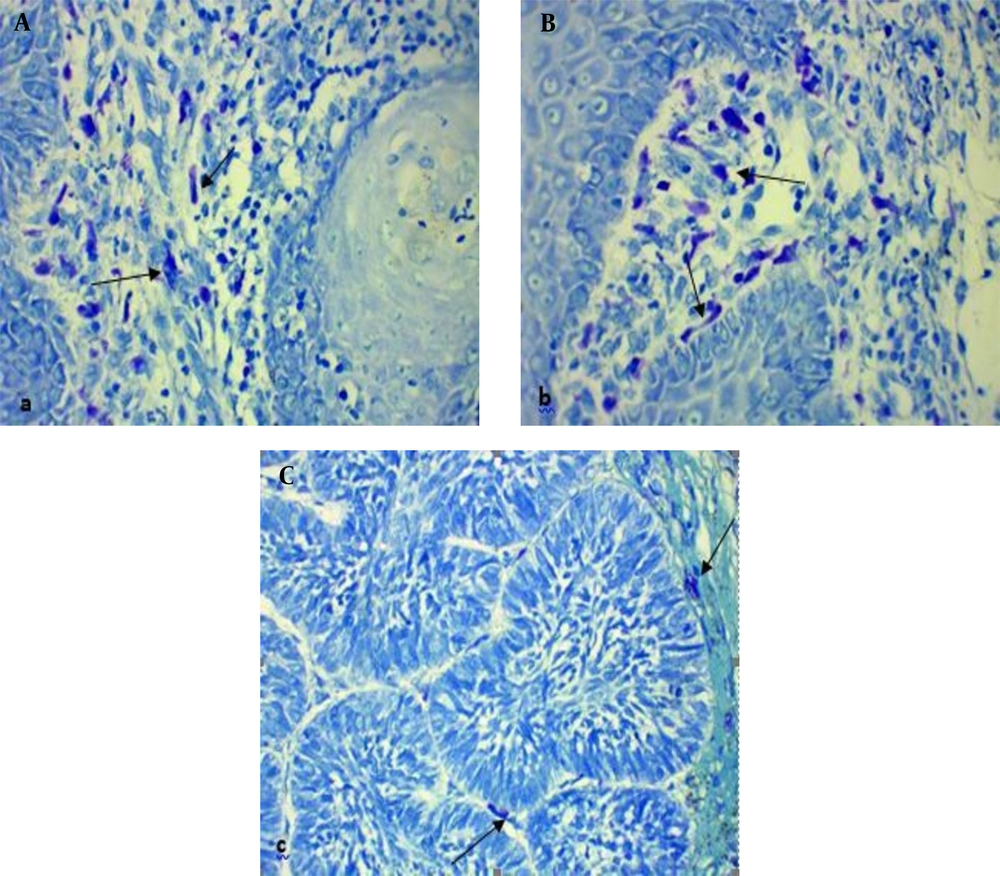
To perform Toluidine blue staining, the samples were placed in potassium permanganate solution for 2 min, washed twice with distilled water and, then, placed in metabisulfite and Toluidine blue solutions for 1 and 5 min, respectively. Finally, the specimens were washed with distilled water and, then, dehydrated with 100% alcohol.
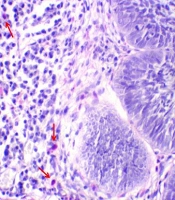
Subsequently, the lamella was stuck on the dried samples and investigated with an optical microscope. To investigate each slide, 10 areas near the tumor islands were randomly selected and studied with a 400 X magnification microscope. The total eosinophil and mast cell counts were recorded as the result for each sample (eosinophil-mast cell/10 HPF). It is worth noting that in this technique, the granules of eosinophils and mast cells were stained red and a purplish red, respectively, and the nuclei appeared light blue.
3.3. Statistical Analysis
The obtained data were coded and, then, described in (absolute and relative) frequency distribution tables, using SPSS (version 21). Furthermore, the data were analyzed through Pearson’s correlation, t test, and ANOVA test. A P-value less than 0.05 was considered statistically significant.
4. Results
4.1. Sample Characteristics
The current study was conducted on 76 samples (40 males and 36 females) obtained from patients with non-melanoma skin cancers with a mean age of 62.97 ± 15.82 years (ranging from 24 to 90). Out of these cases, 46 and 30 samples had BCC and cSCC lesions of the head and neck, respectively. The BCC and cSCC patients had a mean age of 63.74 ± 15.67 (24 - 89) and 61.8 ± 16.25 (33 - 90) years, respectively. The BCC patients were homogenous in terms of gender. However, regarding the cSCC patients, 56.7% of the subjects were male, and others were female.
4.2. Comparison of Immune Cell Count
The mean eosinophil counts were 35.43 ± 35 and 331.27 ± 321.68 eosinophil/10 HPF in the BCC and cSCC samples, respectively (Figure 1). This difference was statistically significant between the two skin cancer types (t test, P = 0.001). The BCC and cSCC samples had mean mast cell counts of 55.33 ± 39.90 and 63.67 ± 40.86 mast cell/10HPF, respectively (Figure 2). Nonetheless, this variable was not significantly different between the two groups (t test; P = 0.380). According to the results, the mean eosinophil in cSCC (Pearson correlation, P = 0.052, r = -0.358) and BCC (Pearson correlation, P = 0.518, r = 0.098) showed no significant relationship with age. In addition, there was no significant relationship between age and the mean mast cell counts in cSCC (Pearson correlation, P = 0.256, r = -0.210) and BCC (Pearson correlation, P = 0.509, r = 0.100). As indicated in Table 1, no significant difference was observed between the two genders in terms of the mean eosinophil and mast cell counts in the cSCC and BCC cases.
aValues are expressed as mean ± SD.
bt test.
In BCC samples, there were 62.5% nodular, 10.9% Sclerosing, 10.9% basosquamous, 8.7% superficial, and 4.3% adenoid types. Nonetheless, there was no subject with other histopathological types of BCC. Additionally, no statistically significant difference was observed between the eosinophil and mast cell counts in the different histopathologic types of BCC (Table 2).
a Values are expressed as mean ± SD.
b ANOVA test.
The mean eosinophil and mast cell counts were higher in cSCC grade III than in other grades. However, this difference was statistically significant only for the mast cells (Table 3). The results of Tukey’s test revealed that the mast cell count was significantly different only between cSCC grade I and grade III (P = 0.041).
a Values are expressed as mean ± SD.
b ANOVA test.
There was a significant correlation between the mean eosinophil and mast cell counts in cSCC (Pearson correlation, P = 0.022, r = -0.418); however, this difference was not statistically significant in BCC (Pearson correlation, P = 0.644, r = 0.070).
5. Discussion
A large amount of eosinophil infiltration has been reported in different tumors, such as oral carcinoma, esophageal malignancy, and prostate and cervical cancer. Eosinophil infiltration plays a positive role in the prognosis of some of these tumors; however, it can have a negative role in this regard in several other cancers (17). During cancer development, eosinophils produce such factors as platelet-derived growth factor (PDGF), IL6, TGFβ, CXCL8, VEGF, b-FGF, and MMP-9, which deliver angiogenic signals to the hypoxic areas of tumors and stimulates the growth of tumor (21).
Rancic et al. reported the presence of eosinophils in the cSCC tumor stroma; however, these cells were not observed in BCC. According to Rancic et al., when the enzymes of eosinophil granules are activated and released, they can contribute to the reduction of the collagen skin barrier and the spread of tumors (22). In the current study, eosinophil count was significantly higher in cSCC than in BCC. Therefore, the presence of eosinophils may be regarded as an effective factor in the biologically invasive behavior of cSCC, compared to that of BCC. On the other hand, the presence of eosinophils is correlated with the prevalence and severity of itching and the severity of pain in cutaneous carcinomas. This relationship is stronger in cSCC than in BCC (23).
In the current study, the mean eosinophil count in the cSCC samples was 331.27 eosinophil/10 HPF, which was higher than the results reported by Duman, using hematoxylin and eosin staining (24). In a study carried out by Meyerholz et al., Sirius red staining (used in the current study) displayed eosinophils with higher contrast and specificity than other staining techniques (i.e., Astra Blue/Vital New Red (AB/VNR), Congo Red, Luna, as well as hematoxylin and eosin) (20).
According to the results of the current study, eosinophil counts were higher in tumors of higher grades. Although this correlation was not statistically significant, it fully supported the findings reported by Duman et al. (24). Eosinophils are capable of producing active 92-kD gelatinase, which breaks down the basement membrane and molecules of the extracellular matrix and has an important role in cSCC invasion (16). Wong et al. demonstrated that the removal of eosinophils could inhibit the development of oral SCC and/or delay it (25). Unlike the mentioned studies, Peurala et al. demonstrated that patients with lip SCC and higher TATE had better survival, compared to those with lower TATE. They also reported that the eosinophil count of more than 4 eosinophil/HPF could result in a more favorable prognosis (26). In the present study, the mean eosinophil count showed no significant correlation with age and gender in cSCC patients. Likewise, Oliveira et al. observed no significant correlation between the age and gender of Oral SCC patients and the presence of eosinophils (27).
Rancic et al. reported the absence of eosinophils in BCC patients; on the other hand, Yosipovitch reported the presence of eosinophils in only 7.2% of BCC patients (22, 23). In the current study, the mean eosinophil count in the BCC patients was 35.43 eosinophil/10 HPF; nevertheless, these cells were not observed in 8.7% of the BCC cases. This discrepancy can be attributed to the staining techniques used by different studies. The role of eosinophils in BCC has been partly elucidated. In this regard, eosinophils migrate to the dermis and produce type 4 collagenase responding to BCC. This process can affect the growth of tumors (15). Our results indicated no correlation between the mean eosinophil count and age, gender, and histopathologic type of BCC patients. To the extent of the researcher’s knowledge, this issue has not been investigated in similar studies.
It has been reported that the mast cells accumulate around and within a large variety of solid cancers. The function of the mast cells in the evolution of a variety of tumors has been recently studied, giving different suggestions, such as shifting the balance in favor of or against tumor growth (7). According to the results of the current study, the mean numbers of the mast cells in cSCC and BCC were obtained as 63.67 ± 40.86 and 55.33 ± 39.90, respectively. However, this difference was not statistically significant.
Similar to the current study, Biswas et al. investigated the role of the mast cells in various skin tumors, including SCC and BCC. They used immunohistochemistry (tryptase) staining to determine the mast cell count. In the mentioned study, the mean number of mast cells was slightly higher in SCC than in BCC (167.22 ± 55.93 for SCC and 166.23 ± 58.23 for BCC); however, this difference was not statistically significant. As a result, the mast cells have a limited role as a prognostic factor in skin tumors. Furthermore, in the study of Biswas et al., the mean number of mast cells was lower in some of the invasive forms of BCC and SCC with higher histopathological grades (28). However, in our study, the mean number of mast cells was significantly higher in grade III than in grade I. This could indicate the role of the mast cells in tumor progression. In line with our results, Parizi et al. and Claudatus et al. reported an increase in the number of mast cells in skin SCC, especially in high-grade types. This finding indicates the role of the mast cells in the progression of these tumors (29, 30). Erkilic and Erbagci investigating the role of the mast cells in BCC, reported an increase in the number of the mast cells in BCC, especially its invasive type (morfea form). They also suggested that the enhancement of the number of the mast cells in BCC may increase the invasive behavior of the tumor and facilitate tumor spread through increasing tumor angiogenesis and collagenase activity in the tumor (31).
Based on the evidence, the mast cells can contribute to matrix degradation and angiogenesis in various tumors due to the release of various mediators, such as heparin, histamine, metalloproteinases, and various growth factors [e.g., fibroblast growth factor, interleukins-6,8,9 (IL-6,8,9), and vascular endothelial growth factor]. Accordingly, the mast cells have been reported to be important in the prognosis of various tumors in gastric, thyroid, bladder, and skin tumors (6, 9).
On the contrary, Jain et al. and Debta et al., using the Toluidine blue method, demonstrated that the increased number of mast cells was associated with a better prognosis of oral SCC. Moreover, they indicated that the mast cells had an antitumor effect. This antitumor effect of the mast cells is exerted by some mediators, such as IL-1, IL-4, and IL-6, which are harmful to tumors and lead to tumor cell apoptosis or production of tumor necrosis factor-alpha by the mast cells, which directly have cytotoxic effect for tumor cells (7, 8).
In addition, the mean number of the mast cells demonstrated no significant correlation with the age or gender of lesions. Consistent with our results, Heidarpour et al. found no significant correlation between age and gender and the number of mast cells in BCC (32). Nevertheless, in a study conducted by Debta et al., a significant relationship was observed between the number of mast cells in SCC and age (33). In a study performed by Parizi et al., there was a significant correlation between the number of mast cells in SCC and gender; in this regard, a higher number of mast cells were observed in females (29).
There are some limitations in our study that include the small sample size, retrospective nature of the study, and newer diagnostic modalities were not used for quantification of tissue cells.
Therefore, it is essential to perform further studies for a better understanding of the activation mechanisms, immunomodulation capacity of these inflammatory cells, and their exact role in the invasive behavior of cancers. This can open new perspectives about the future therapeutic strategies targeted to these multifunctional cells.
5.1. Conclusions
Considering the significantly higher number of eosinophils in cSCC than in BCC, it may be concluded that eosinophils are among the factors that mostly account for the biologically and clinically invasive behaviors of cSCC, compared to those of BCC. The number of mast cells with a slight difference in cSCC was greater than that of BCC. Therefore, the mast cells cannot be considered a prognostic factor responsible for the different behaviors of these two tumors.
In addition, it seems that the clinicopathological factors, such as age or gender, did not affect the mean eosinophil and mast cell counts in cSCC and BCC patients. Given the low number of studies conducted on the role of eosinophils and mast cells in cSCC and BCC, it is essential to perform further studies to obtain knowledge regarding the detailed mechanism of these inflammatory cells.