1. Background
The studies highlighted a role for microbiome dysbiosis in the progression and development of cancer. Colorectal cancer (CRC) patients have different characteristics of colon bacteria than healthy individuals. Alteration of intestinal microbiota in CRC patients leads to increased cell proliferation compared to intestinal microbiota in healthy individuals (1). Although changes in bacterial composition in CRC patients are well studied, the archaea composition in CRC has been rarely studied (2). Archaea have different cellular characteristics than bacteria and eukaryotes, such as the lack of peptidoglycan in the cell wall and the membrane formed by L-glycerol ethers/isoprenoid chains (3, 4). Some archaea have a unique metabolism called methanogenesis, which is done by methane-producing archaea (methanogens). Methanogens are found in the human gut mucosa (5), vaginal mucosa (6), oral cavity (7), and skin (8). Methanogens grow by producing methane gas under anaerobic conditions. Methanogens have been linked to intestinal dysbiosis, but there have been no reports of direct involvement in their pathogenesis using toxins or virulence factors. Most of the methanogens in the human gut can use hydrogen to reduce carbon dioxide to methane, so-called hydrogenotrophs (9); they use hydrogen produced by neighboring microbes as a substrate for methane production (10). Even though the number and variety of archaea in the human gut is distant less than bacteria, their importance in human health and disease can't be ignored. Archaea diversity is usually influenced by diet, age, the effects of other microorganisms, and the physiology of the human gut; this archaea diversity may be involved in intestinal dysbiosis (11).
There are some reports of the association of archaea with some human diseases. Methanogens are related to infectious diseases such as periodontal disease (12), obesity (13), anaerobic abscesses, peri-implantitis, anorexia, and inflammatory bowel disease (14-16). It has recently been reported that alteration in the composition of gut archaea and interaction with bacteria are associated with colorectal cancer (17, 18). The studies in this field are very limited, no studies have been performed in Iran to examine gut archaea in patients with CRC. To this day, our knowledge of gut archaeome is extremely limited because of the unculturable nature of those microorganisms.
2. Objectives
The aim of this study was to estimate the differences in archaeome communities between CRC samples and healthy controls (HC) through high-throughput 16S rRNA sequencing, as well as to compare archaea composition by age and gender in CRC and HC individuals.
3. Methods
3.1. Biopsy Sampling
Biopsy samples were collected from individuals referred to the colonoscopy ward of Imam Ali Research Hospital affiliated with Zahedan University of Medical Sciences between June 2019 and January 2020. We collected biopsy samples of the colon mucosal tissue from 17 CRC patients and 13 healthy controls (HC). The exclusion criteria were familial or hereditary colorectal adenoma or tumor, inflammatory bowel disease, irritable bowel syndrome, use of antibiotics and probiotic products within two months before sampling.
3.2. Microbial DNA Extraction and Amplification of 16S rDNA Gene
Microbial DNA from biopsy samples was extracted using NucleoSpin Microbial DNA Mini kit (MN, Germany) according to the manufacturer’s instructions. DNA quality and quantity were assayed, then stored at -20°C for further analysis. Table 1 shows the clinical characteristics of the samples and the bacterial DNA concentration extracted from each colon biopsy sample in CRC patients and healthy individuals. We amplified the microbial DNA by PCR using 515F (GTGCCAGCMGCCGCGGTAA) and 806R (GGACTACHVGGGTWTCTAAT) (19) primers (metabion, Germany) for targeting the variable region of 16S rDNA gene V4. 2x PCR Master Mix RED (Ampliqon, Denmark) was used for PCR. Amplification was performed using a Thermal Cycler (Bio-Rad, United States) with the following conditions: initial denaturation of 94°C for 3 min; followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min; and a final extension at 72°C for 10 min. The relative quality of PCR products was determined by the run on 2% agarose gel electrophoresis (Figure 1A), then were purified (Expin PCR SV-mini, Gene All), and final products within the DNAstable tubes were sent to Macrogen Company (South Korea) for determining the quality control (Figure 1B) and high-throughput sequencing on the 16S rRNA illumina platform paired-end sequencing (Illumina sequencer, Macrogen).
| CRC | HC | P Value | |
|---|---|---|---|
| Number | 17 | 13 | |
| Age | 50.76 ± 14.6 | 47.7 ± 11.1 | 0.534 |
| Age range | 32 -72 | 35 -75 | |
| Gender | 0.748 | ||
| Male | 10 | 6 | |
| Female | 7 | 7 | |
| Gender by age | |||
| Male | 0.696 | ||
| > 50 | 6 | 3 | |
| < 50 | 4 | 3 | |
| Female | 0.554 | ||
| > 50 | 3 | 1 | |
| < 50 | 4 | 6 | |
| BMI | 25.8 ± 3.2 | 24.1 ± 2.7 | 0.134 |
| DNA concentration extracted from each sample (ng/uL) | 18.7 ± 6.4 | 16.5 ± 6.6 | 0.373 |
Abbreviations: CRC, colorectal cancer; HC, healthy controls; BMI, body mass index.
3.3. Bioinformatics Sequencing Data Analysis
Analysis of 16S archaea sequence data was performed using QIAGEN CLC Genomics Workbench (v.21.0.4) software. Raw sequences data in options of Paired-end were filtered with readings of 200 to 550 lengths. Next, the adapter was trimmed from Illumina sequences. In the next step, the Paired-end reads were clustered into operational taxonomic units (OTUs) with 97% sequence similarity (20). To enhance the visualization of the OTU abundance table, we decorated it with a metadata file to see results based on different groups. We examined differential abundance taxa analysis to detect differences in classification composition between HC and CRC patients.
3.4. Statistical Analysis
Clinical characteristics of the samples data were analyzed with IBM SPSS 23.0 version. Quantitative data with a normal distribution are expressed as the mean ± standard deviation. The difference between groups was analyzed using a t-test. Gender by age data was compared with a chi-square test. P < 0.05 was considered statistically significant.
4. Results
4.1. Archaeome Composition in the CRC and HC Groups
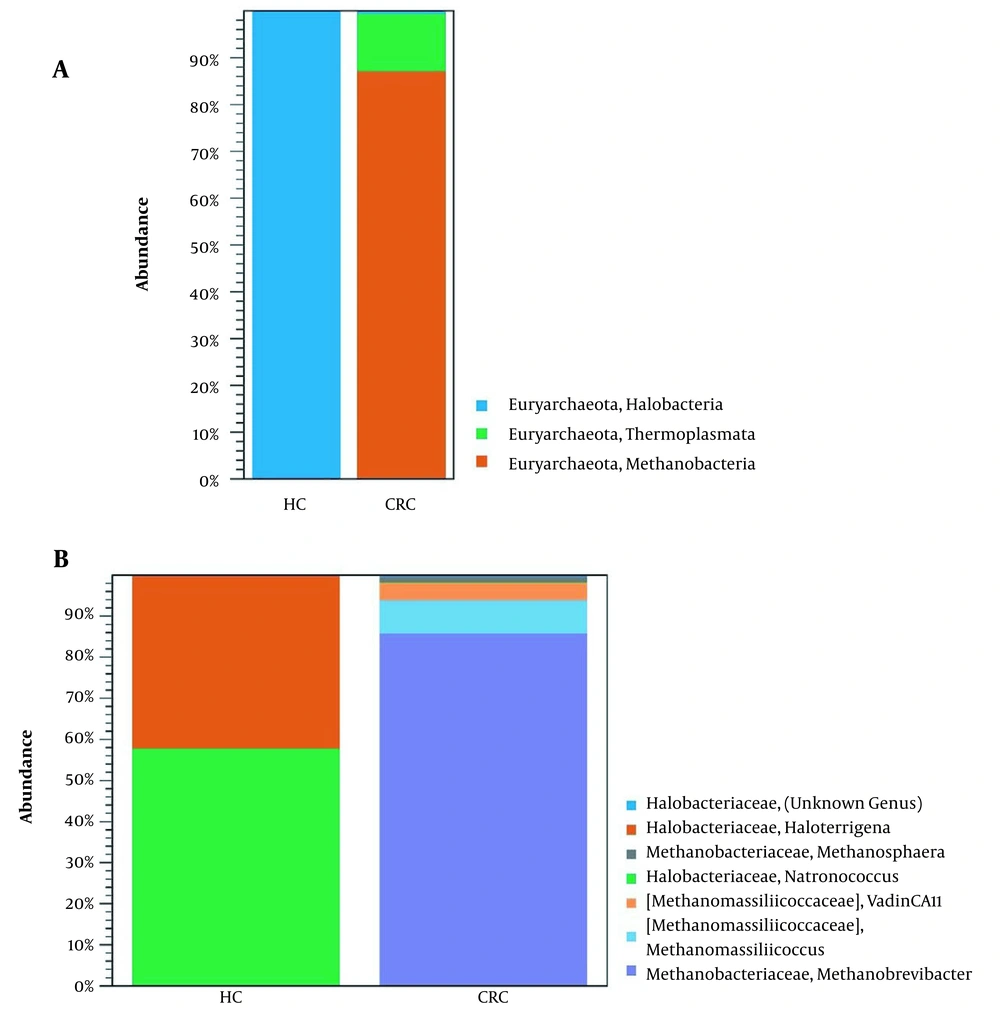
Our analysis showed that archaea OTUs clustered into one phylum, three classes, three orders, three families, and seven genera. The two groups contain seven archaea at the genus level: Methanobrevibacter, Methanomassiliicoccus, VadinCA11, Natronococcus, Haloterrigena, Methanosphaera, and Halobacteriaceae (unclassified genus). In the CRC patients, Methanobrevibacter (86%) and Methanomassiliicoccus (8%) were overrepresented at the genus level, while in the HC group only two genera Natronococcus (58%) and Haloterrigena (42%) were observed (Figure 2). The results of differential abundance taxa analysis showed significant differences between HC and CRC patients (Table 2). The results of the CRC group versus HC group- fold change were significant (P ≤ 0.05). Table 3 shows archaea OTUs distribution (after adapter trimming) between CRC and HC groups.
| Taxonomy | CRC vs. HC - Max Group Mean | CRC vs. HC - Fold Change | CRC vs. HC - P-Value | CRC vs. HC - FDR P-Value |
|---|---|---|---|---|
| Archaea; Euryarchaeota; Methanobacteria; Methanobacteriales; Methanobacteriaceae; Methanobrevibacter | 224.764 | 1992.96 | 1.473E-12** | 2.21605E-10 |
| Archaea; Euryarchaeota; Thermoplasmata; E2; [Methanomassiliicoccaceae]; Methanomassiliicoccus | 22.117 | 216.67 | 1.31382E-06** | 1.77006E-05 |
| Archaea; Euryarchaeota; Thermoplasmata; E2; [Methanomassiliicoccaceae]; VadinCA11 | 7.353 | 67.33 | 0.0001** | 0.0008 |
| Archaea; Euryarchaeota; Halobacteria; Halobacteriales; Halobacteriaceae; Natronococcus | 5.615 | -5.61 | 0.03* | 0.05 |
| Archaea; Euryarchaeota; Halobacteria; Halobacteriales; Halobacteriaceae; Haloterrigena | 4.076 | -24.88 | 0.0009** | 0.003 |
| Archaea; Euryarchaeota; Methanobacteria; Methanobacteriales; Methanobacteriaceae; Methanosphaera | 3.411 | 22.77 | 0.003** | 0.009 |
| Archaea; Euryarchaeota; Halobacteria; Halobacteriales; Halobacteriaceae(unknown genus) | 0.588 | 5.83 | 0.06 | 0.1 |
Abbreviations: CRC, colorectal cancer; HC, healthy controls.
a*P < 0.05, **P < 0.01.
| Taxonomy | Combined Abundance | Mean | Std | HC Abundance | CRC Abundance |
|---|---|---|---|---|---|
| Archaea; Euryarchaeota; Halobacteria; Halobacteriales; Halobacteriaceae | 10 | 5 | 7.07 | 0 | 10 |
| Archaea; Euryarchaeota; Halobacteria; Halobacteriales; Halobacteriaceae; Haloterrigena | 53 | 26.5 | 37.4 | 53 | 0 |
| Archaea; Euryarchaeota; Halobacteria; Halobacteriales; Halobacteriaceae; Natronococcus | 83 | 41.5 | 44.5 | 73 | 10 |
| Archaea; Euryarchaeota; Methanobacteria; Methanobacteriales;Methanobacteriaceae; Methanobrevibacter | 3928 | 1964 | 2777.5 | 0 | 3928 |
| Archaea; Euryarchaeota; Methanobacteria; Methanobacteriales; Methanobacteriaceae; Methanosphaera | 58 | 29 | 41.01 | 0 | 58 |
| Archaea; Euryarchaeota; Thermoplasmata; E2; [Methanomassiliicoccaceae]; Methanomassiliicoccus | 376 | 188 | 265.8 | 0 | 376 |
| Archaea; Euryarchaeota; Thermoplasmata; E2; [Methanomassiliicoccaceae]; VadinCA11 | 191 | 95.5 | 135.05 | 0 | 191 |
Abbreviations: CRC, colorectal cancer; HC, healthy controls; Std, standard deviation.
4.2. Archaeome Composition in the CRC and HC Groups Based on Gender
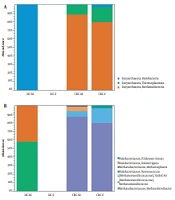
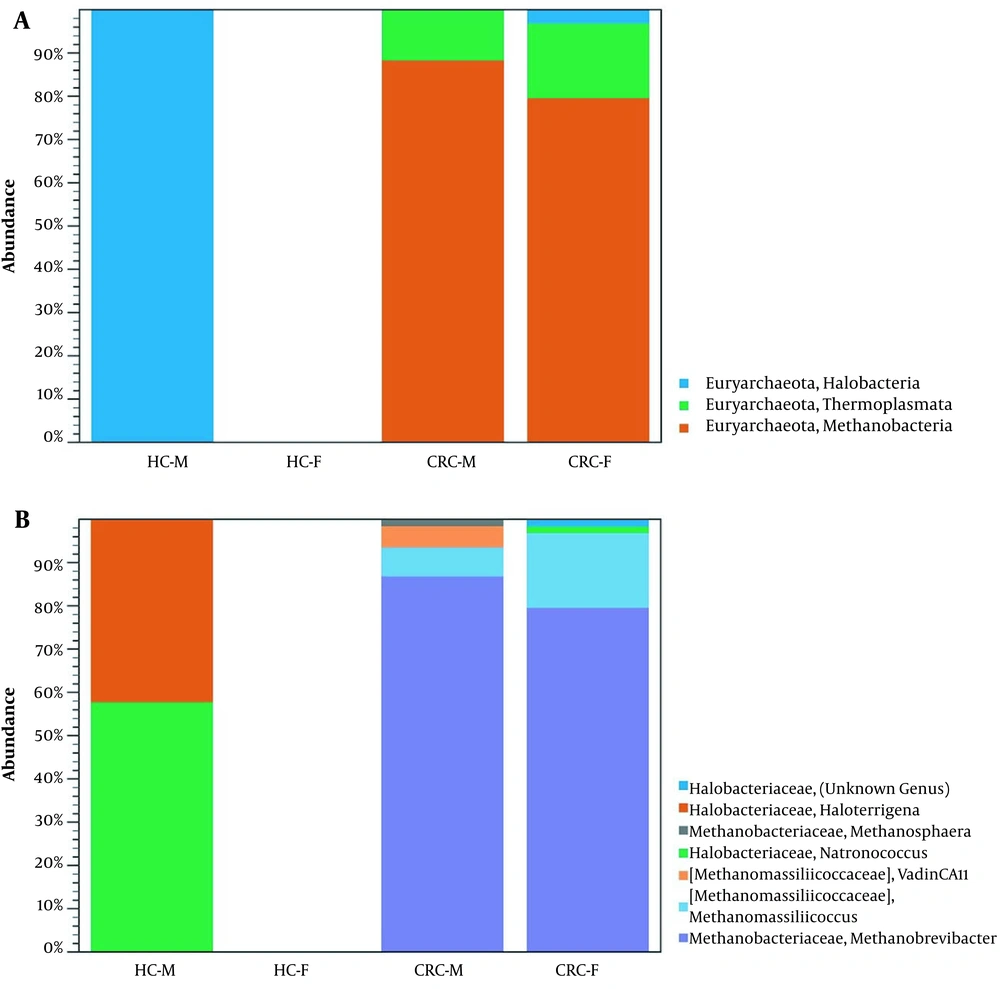
We compared the archaea composition in four groups: CRC-F (female), CRC-M (male), HC-F, and HC-M. Our analysis couldn't find any archaea in the HC-F group (Figure 3). Two classes Methanobacteria and Thermoplasmata were enriched in CRC-F and CRC-M groups, while Halobacteria were enriched in the HC-M group. Methanobrevibacter (87% and 80%) in the CRC-M and the CRC-F, and Natronococcus (58%) in the HC-M had the highest relative abundance. The results revealed that the diversity of archaea was higher in men than women in both the CRC patient and HC groups.
4.3. Archaeome Composition in the CRC and HC Groups Based on Age
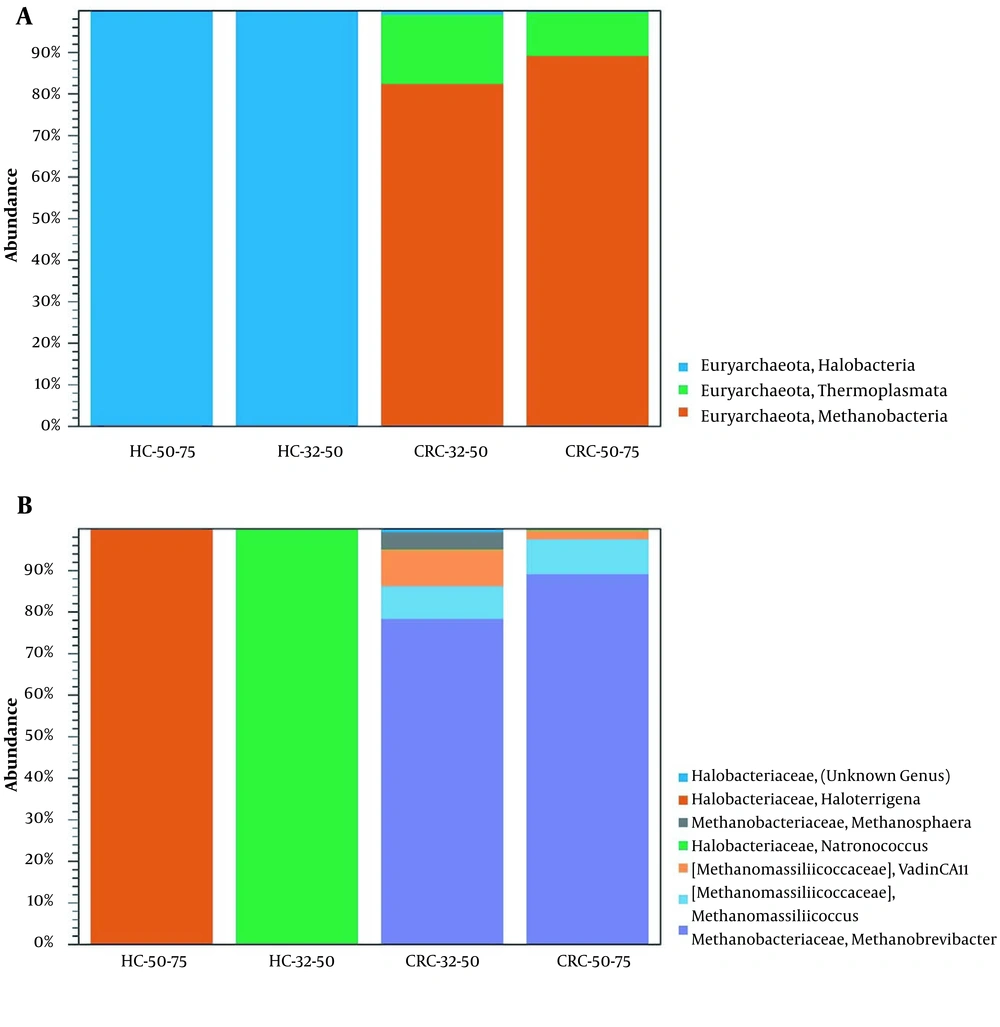
We compared the archaea composition by age (HC-32-50, HC-50-75, CRC-32-50, and CRC-50-75) into four groups. Methanobrevibacter (89% and 79%) and Methanomassiliicoccus (8% and 8%) in the CRC-50-75 and the CRC-32-50 were overrepresented, respectively (Figure 4). The two genera Natronococcus and Haloterrigena were present only in HC-32-50 and HC-50-75 groups, respectively. Our results showed that six genera in the CRC-32-50 group are present and the diversity of archaea in the CRC-32-50 group is higher than the other groups.
Relative abundance of archaeome composition in CRC patients and HC group based on age at (A) class, and (B) genus levels. HC-32-50, healthy control 32 to 50 years old; HC-50-75, healthy control 50 to 75 years old; CRC-32-50, colorectal cancer 32 to 50 years old; CRC-50-75, colorectal cancer 50 to 75 years old.
5. Discussion
Our results showed that methanogens are the most abundant archaea in the colon of CRC patients. Food particles are broken down in the human gut by fermenting bacteria, as a result, products such as short-chain fatty acids (SFA), CO2, and H2 are produced. Methanogens gain energy by reducing CO2 to methane, with H2 as the primary electron donor (21). Methanogenic archaea are identified in the range of 25 - 40% in children and 42 - 82% in adults (22). High levels of breath methane has been observed in patients with malignant polyps and CRC (23). Although methane is not carcinogenic, however, methane oxidation forms formaldehyde, which is carcinogenic (24). In addition, it has been shown that hydrogen sulfide gas can cause angiogenesis and, as a genotoxic, inhibit DNA repair (25). Several factors affect the presence of methanogens in the human gut, including ethnicity, geography, host genetics, diet, age, and microbial composition (2, 26).
Although the mechanism of methanogens in CRC is still questionable, it can play pathogenic role in carcinogenesis of colorectal cancer (16, 17, 27, 28). CRC can not only be attributed to a particular pathogen but also with a microbial shift towards an anaerobic consortium, consisting of saccharolytic and proteolytic anaerobic bacteria, including Clostridium, Eubacterium, the Bacteroides/Prevotella cluster, the terminal-degrading methanogens, and sulfate-reducing bacteria, with an attendant reduction in probiotic bacteria (21, 29). Methanogens in the human gut are mainly dependent on hydrogen, for the reduction of CO2 and the reduction of methyl compounds: Depletion of hydrogen by methanogens optimizes fermentation and modifies the metabolic pathways of fermentative bacteria (30). Fermentation of polysaccharides by colonic bacteria can produce short-chain fatty acids (SCFAs) such as butyrate. Patients with CRC have been shown to have an increased level of methanogens and decreased butyrate levels (31). Butyrate provides energy for gut epithelial cells, regulates host immune system and mucin production, upregulate host immune system and mucin production, alter toxic or mutagenic compounds, and reduce the size and number of crypt foci, which are abnormal glands in intestinal epithelia that lead to colorectal polyps (32, 33).
According to our results, Methanobrevibacter and Methanomassiliicoccus were overrepresented in the CRC patients at the genus level. By targeting the mcrA gene using PCR, the researchers examined the relative prevalence of methanogens in health and diseases such as CRC. Their study showed that 45% of CRC patients contain methanogenic genera (34). Opposite to our results, a study in 2020 appeared that the fecal samples from patients with CRC had enrichment of halophilic and depletion of methanogenic archaea (17). The reason for these different results was probably a difference in the sampling location, they were sampled from the stool and we were sampled from the colon. The most common genera of methanogens are the closely related Methaonbrevibacter and Methanosphaera. Methanobrevibacter, that previously called Methanobacterium, was first isolated from human feces in 1968 (35). Methanobriobacter stadtmanae secrete potent proinflammatory cytokines (TNF-a, IL-1b) from the monocyte-derived dendritic cells in patients with inflammatory bowel disease (36). Methanogens can employ virulence strategies similar to anaerobic bacteria in humans (37). Methanogens convert heavy metals and metalloids into methylated derivatives, including trimethylbismuth which is toxic for both human and bacterial cells (38). According to previous information about the pathogenicity of methanogens and considering the significant fold change of methanogens in CRC patients, it can be concluded that methanogens may be involved in the development of CRC.
Our results showed the presence of members Halobacteriaceae including Natronococcus and Haloterrigena in the colon of healthy individuals. In the past, low salt concentrations in the human intestine were thought to be insufficient to survive halophilic archaea. Still, there have been reports that there are halophilic archaea that can survive in salt concentrations close to seawater (39, 40). Biopsy of the colon and fecal samples of patients showed that halophilic archaea were present in the gastrointestinal tract as part of the mucosal microbiota (41). In addition, analysis of fecal samples from a Korean population showed the presence of halophilic archaea (42). A recent study in the Korean population of healthy individuals identified a variety of haloarchaea (43). However, the question is: what are haloarchaea doing in the colon? The presence of haloarchaea in the gut may be transient; they may have entered the gastrointestinal tract through food (40). Another possibility is that halophilic archaea may be members of the human colon flora (41), although the colon environment is not a saline environment. Some members of the Halobacteriaceae family survive in low-salt environments (~ 150% mM) (44). Given that the intestinal environment of healthy individuals has a medium salinity similar to plasma (135 - 145 mM sodium) the existence of this group of archaea is justified. However, the role of haloarchaea in human health or disease is still unclear and needs further study in the future.
We have found that Methanobriobacter is more common in CRC patients over 50 years of age. During the aging process, the density of methanogens in the human colon may increase (45). Our results showed that the diversity of archaea in the CRC-32-50 group is higher than the other groups. Studies have reported that methanogens are more diverse in the human gut in the age range of 20 - 60 years (38). In addition, our results showed that the members of the archaea were more common in men than women. Previous human studies have shown sex-related differences in the intestine microbiota (46, 47). The reason for this difference in archaea composition can be the following factors: sex hormones, drugs, diet, and body mass index (48, 49).
5.1. Conclusions
The human gut archaeome of CRC patients and HC is different, and the level of methanogens in CRC patients is higher than the HC. High relative abundance of archaeal methanogens in CRC patients may be strongly with the development of CRC.