1. Background
For patients with breast cancer, breast conserving surgery (BCS) followed by adjuvant radiotherapy (RT) is taken into account as the most common treatment method (1-3). Literature has shown that the number of females with breast cancer has been increased during the recent two decades around the world (4-6). In this regard, a large number of studies have shown that adjuvant RT of the mentioned patients may improve local control and overall survival of them (6-9). The RT of the patients is commonly done, using two-photon tangential beams, which irradiate the whole breast and the anterior part of the thoracic volume (6, 10, 11). A wealth of studies have demonstrated that RT of the patients may increase the normal tissue complications, including, lung secondary cancer, and also heart morbidity and mortality, which are resulted from imposed radiation dose to cardiovascular structures (7, 12-17). Zablotska and Neugut have reported that the imposed radiation dose to the lungs may pose a moderate rise in complications for the mentioned organ (12). There are different RT modalities such as 3D-CRT and IMRT tangential plans, which are commonly applied to treat the mentioned patients. A host of studies have discussed the imposed dose to the organ at risk (OARs), using different modalities (17-20). Aznar et al. have found that the evaluation of the radiation dose to the whole heart, arch, and whole LAD is a suitable approach, because the calculation of radiation dose to only one of these structures may lead to imposing unnecessary doses on them and also may increase the risk of cardiac complications (17). Zhang et al. have found that IMRT may provide higher target dose coverage and dose uniformity compared to conformal RT for patients with left-sided breast cancer (19).
Although many studies have demonstrated that using tangential beams may provide enough dose distribution in the target volumes compared to other methods, data about depth dose distribution of above stated tangential methods and their complications on the normal tissues such as ipsilateral lung, heart, and cardiovascular structures including, LAD, and also RCA are scarce. According to different studies, it would seem that there are no exact advantages and disadvantages of 3D-CRT, IMRT, and hypofractionated RT concerning their adverse effects on coronary arteries such as LAD and RCA.
2. Objectives
This work aimed at evaluating the dose distribution, and also radiobiological models including tumor control probability (TCP) and normal tissue complications probability (NTCP) for target volume and normal tissues including ipsilateral lung, heart, and cardiovascular structures namely, LAD and RCA in the above-mentioned tangential plans.
3. Methods
The study protocol was approved by the ethical board of Isfahan University of Medical Sciences, Isfahan, Iran (IR.MUI.MED.REC.1399.677) according to the 1975 Helsinki declaration and its revision in 2000.
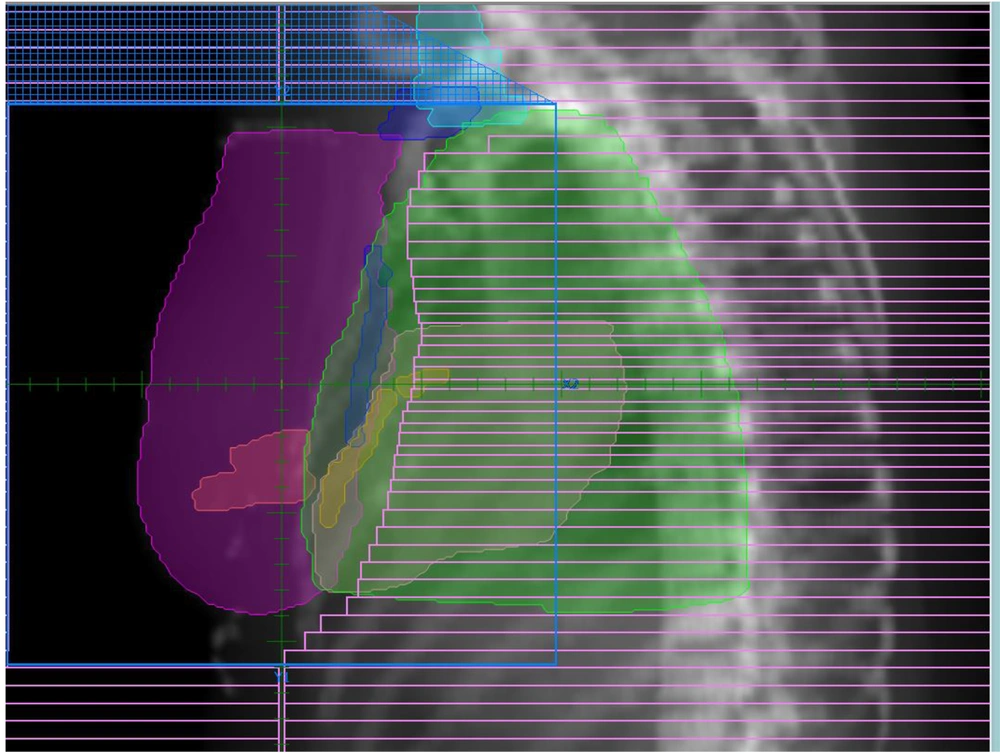
Eighty patients with left-sided breast cancer (N1T1 – N3T3), who underwent RT in Sayed-Al Shohada Hospital, Isfahan, Iran were chosen to participate in this study. The patients were divided into two groups (Table 1) including BCS (n = 50) and post-mastectomy patients (n = 30). CT simulation images of the patients were imported on the treatment planning software (TiGRT, LinaTech, China). Treatment plans of studied modalities including 3D-CRT, 6fields (6FIMRT), 9fields IMRT (9FIMRT) inverse plans, and also hypofractionated IMRT for each patient were done by 3 independent expert radiation oncologists, using the mentioned TPS. The used TPS was similar for IMRT and hypofractionated plans. The applied TPS was commissioned according to a Siemens Primus linear accelerator measured data (Siemens, Germany). Also, 2 automated collimators were used in the mentioned linac. Treatment planning of the patients was performed, using 6 MV photon beams of the stated linear accelerator according to the patient’s geometry and chest wall sizes. In this study, the chest wall surface, 3 levels of the axilla, and supraclavicular lymph nodes were defined as CTV. Furthermore, the planning target volume (PTV) was defined with a 1 cm margin around the CTV. Moreover, the OARs were ipsilateral lung, heart, LAD, and also RCA. The prescribed dose for the 3D-CRT and IMRT inverse plans was 2 Gy per fraction (25 fractions) for PTV with a total dose of 50 Gy, while the dose for the hypofractionated RT was 2.3 Gy per fraction (20 fractions) with the total prescribed dose of 46 Gy. The source to surface distance (SSD) was 100 cm, and the above plans were done based on SSD set up for each patient. The treatment plans were done according to RTOG protocol and the whole chest wall was irradiated by 6MV tangential photon beams. Furthermore, the mean imposed radiation dose to the OARs was measured with and without internal mammary nodes for each patient. In addition, the volume of the heart, LAD, and RCA, which were irradiated more than 25 Gy (V25Gy), and also the volume of the ipsilateral lung which was exposed to more than 20 Gy (V20Gy) were compared for each patient among the mentioned modalities. Moreover, the maximum heart distance (MHD) was measured, using beam eye view (BEV). Figure 1 illustrates the BEV for one of the studied patients.
| Female (100%) | Male | |
|---|---|---|
| Age, mean ± SD | 53.45 ± 8.21 year (35 - 80) | - |
| T stage | T1-T3 | - |
| N stage | N1-N3 | - |
| M stage | M0: 77.5 % (n = 62) ; M1: 22.5% (n = 18) | - |
| BCS | 62.5% (n = 50) | - |
| Mastectomy | 37.5% (n = 30) | - |
Abbreviation: BCS, Breast conserving surgery.
3.1. TCP-NTCP Modeling
The TCP and NTCP for each of the above-mentioned situations were assessed, using DVH data. In this study, Poisson linear-quadatric (PLQ) model was employed for estimating the TCP according to Equation 1 (21, 22):
Where N is the initial number of tumoral cells, and ps (D) is the cell survival fraction after a dose D.
For NTCP calculation, Lyman-Kutcher-Burman (LKB) model was used according to Equation 2 (23, 24).
Where t is calculated based on the following formula (Equation 3):
In the above equation, "m" is the maximum inverse slope of the dose-response curve, EUD is the equivalent uniform dose of the mentioned OARs (25-29), and also D50 is the total imposed radiation dose to the studied normal tissues that may lead to force 50% complication to the organ.
3.2. MATLAB Program & R Environment
The generated DVHs were evaluated and the TCP-NTCP models were created in MATLAB and R programs. The α/β ratio of OARs, the slope of the dose-response curve of 50%, dose tolerance of 50%, and m were determined based on PLQ- LKB models in the mentioned programs.
4. Results
Tables 2 and 3 indicate the mean (SD) dose to PTV and also imposed radiation dose to the OARs among the studied methods for BCS and post-mastectomy patients. Table 4 compares TCP and OARs complications probabilities for both BSC and mastectomy patients. PTV coverage of 9FIMRT and hypofractionated IMRT (95%) was higher than the 6 fields (94%) and 3D-CRT (92%).
| 3D-CRT (With/Without Internal Mammary Fields) | 6fiels IMRT Inverse Planning (With/Without Internal Mammary Fields) | 9fields IMRT Inverse Planning (With/Without Internal Mammary Fields) | Hypofractionated IMRT (With/Without Internal Mammary Fields) | 3D-CRT vs. 6FIMRT (P-Value) | 3D-Crtvs. 9FIMRT (P-Value) | 3D-CRT vs. Hypofractionated IMRT (P-Value) | 6F vs. 9FIMRT (P-Value) | 6F vs. Hypofractionated IMRT (P-Value) | 9F vs. Hypofractionated IMRT (P-Value) | |
|---|---|---|---|---|---|---|---|---|---|---|
| PTV coverage (%) | 92 | 94 | 95 | 95 | - | - | - | - | - | - |
| Ipsilateral lung | ||||||||||
| Mean dose | 12.62 ± 0.56/ 12.21 ± 0.43 | 8.32 ± 1.03/ 8.04 ± 0.87 | 10.57 ± 1.01 / 9.91 ± 0.74 | 10.23 ± 1.00 / 9.81 ± 0.78 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Maximum dose | 58.77/54.46 | 31.58 /27.29 | 37.64 / 32.46 | 36.25 / 31.73 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Minimum dose | 42.21/ 41.08 | 29.37/ 27.34 | 34.86/31.46 | 33.67/31.62 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| V20Gy (%) | 12.23 ± 1.93/10.08 ± 1.07 | 9.46 ± 1.26/7.43 ± 1.14 | 10.48 ± 1.41/8.15 ± 1.38 | 10.31 ± 1.22 /8.07 ± 1.23 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Heart | ||||||||||
| Mean dose | 4.95 ± 0.86 /2.26 ± 0.72 | 2.53 ± 1.18 /2.36 ± 0.69 | 3.62 ± 1.18 / 4.51 ± 0.84 | 3.37 ± 1.07 / 4.26 ± 0.84 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Maximum dose | 55.04 /48.02 | 44.24/ 41.78 | 47.56/43.61 | 47.32/43.16 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Minimum dose | 0.22 /0.19 | 0.20/ 0.18 | 0.20/ 0.19 | 0.20/ 0.17 | > 0.005 | > 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| V25Gy (%) | 21.43 ± 1.87/18.51 ± 2.14 | 22.07 ± 1.27/ 13.76 ± 1.04 | 22.98 ± 0.28/ 14.29 ± 1.37 | 21.47 ± 0.23/ 14.03 ± 1.16 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| LAD | ||||||||||
| Mean dose | 6.79 ± 1.25 /4.12 ± 0.87 | 3.14 ± 1.37 / 3.93 ± 0.95 | 5.46 ± 1.83 /4.38 ± 1.04 | 5.24 ± 1.15 /4.24 ± 0.97 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Maximum dose | 56.21/ 52.14 | 45.23/ 49.61 | 47.65/50.19 | 46.17/50.02 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Minimum dose | 1.26/ 1.15 | 0.76/ 0.50 | 1.08/0.98 | 1.01/0.89 | > 0.005 | > 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| V25Gy (%) | 22.56 ± 2.37/19.27 ± 1.38 | 17.63 ± 2.41/15.75 ± 1.24 | 18.56 ± 1.51/17.27 ± 0.64 | 18.23 ± 1.27/ 16.97 ± 0.32 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| RCA | ||||||||||
| Mean dose | 1.17 ± 0.06 /0.73 ± 0.18 | 0.71 ± 0.23/ 0.58 ± 0.27 | 0.92 ± 0.14/ 0.89 ± 0.11 | 0.89 ± 0.10/ 0.82 ± 0.11 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Maximum dose | 43.18 / 35.63 | 38.64 / 37.69 | 40.94 / 39.77 | 40.05 / 39.21 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Minimum dose | 0.15/ 0.11 | 0.10/0.10 | 0.11/ 0.11 | 0.09/ 0.08 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| V25Gy (%) | 16.42 ± 1.59 / 13.74 ± 1.32 | 14.53 ± 1.24 / 10.36 ± 1.57 | 14.79 ± 1.64 / 10.98 ± 1.06 | 14.34 ± 1.16 / 10.24 ± 1.11 | > 0.005 | > 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Contralateral breast | ||||||||||
| Mean dose | 3.36 ± 0.42/ 2.21 ± 0.21 | 2.12 ± 0.87/ 1.94 ± 0.23 | 2.57 ± 0.34 / 2.07 ± 0.14 | 2.41 ± 0.31 / 2.03 ± 0.11 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Maximum dose | 5.25/4.27 | 4.58 /3.29 | 4.93 / 3.76 | 4.58 / 3.49 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Minimum dose | 2.43/ 2.08 | 2.17/ 1.63 | 2.31/1.91 | 2.18/ 1.82 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| V20Gy (%) | 8.23 ± 0.94 /7.57 ± 0.74 | 6.85 ± 1.16 / 6.12 ± 0.94 | 7.15 ± 1.07 / 6.54 ± 0.83 | 6.99 ± 0.86/ 6.21 ± 0.46 | > 0.005 | > 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| MHD | ||||||||||
| Mean (range) | 1.26 (0.9- 1.43) | 1.26 (0.9- 1.43) | 1.26 (0.9- 1.43) | 1.26 (0.9- 1.43) | - | - | - | - | - | - |
Abbreviations: BCS, breast conserving surgery; 3D-CRT, 3-dimensional conformal radiotherapy; IMRT, intensity modulated radiotherapy; RCA, right coronary artery; LAD, left ascending coronary artery
| 3D-CRT (With/Without Internal Mammary Fields) | 6fiels IMRT Inverse Planning (With/Without Internal Mammary Fields) | 9fields IMRT Inverse Planning (With/Without Internal Mammary Fields) | Hypofractionated IMRT (With/Without Internal Mammary Fields) | 3D-CRT vs. 6FIMRT (P-Value) | 3D-Crtvs. 9FIMRT (P-Value) | 3D-CRT vs. Hypofractionated IMRT (P-Value) | 6F vs. 9FIMRT (P-Value) | 6F vs. Hypofractionated IMRT (P-Value) | 9F vs. Hypofractionated IMRT (P-Value) | |
|---|---|---|---|---|---|---|---|---|---|---|
| PTV coverage (%) | 92 | 94 | 95 | 95 | - | - | - | - | - | - |
| Ipsilateral lung | ||||||||||
| Mean dose | 15.37 ± 0.31/ 15.11 ± 0.34 | 11.74 ± 1.08/ 11.04 ± 0.34 | 13.41 ± 1.18 / 12.39 ± 0.63 | 12.24 ± 1.12 / 11.41 ± 0.39 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Maximum dose | 61.32/57.27 | 34.85 /29.84 | 40.21 / 43.20 | 39.43 / 34.52 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Minimum dose | 45.14/ 44.21 | 32.21/ 30.26 | 33.51/32.61 | 32.38/31.89 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| V20Gy (%) | 14.61 ± 1.56/12.17 ± 1.08 | 12.57 ± 1.17/10.57 ± 1.26 | 13.25 ± 1.23/ 11.15 ± 1.07 | 12.99 ± 1.17/10.99 ± 1.41 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Heart | ||||||||||
| Mean dose | 7.95 ± 0.28 /5.64 ± 0.54 | 5.32 ± 1.18 /5.14 ± 0.69 | 6.28 ± 1.09 / 6.11 ± 0.73 | 6.10 ± 0.99 / 6.07 ± 0.57 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Maximum dose | 58.14 /51.33 | 47.54/ 44.39 | 50.14/46.28 | 50.43/46.25 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Minimum dose | 0.26 /0.21 | 0.23/ 0.19 | 0.23/ 0.19 | 0.23/ 0.18 | > 0.005 | > 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| V25Gy (%) | 24.36 ± 1.17/21.35 ± 2.73 | 25.01 ± 1.08/16.54 ± 1.14 | 25.88 ± 0.24/17.39 ± 1.17 | 24.47 ± 0.29/17.04 ± 1.11 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| LAD | ||||||||||
| Mean dose | 9.83 ± 1.25 /7.25 ± 0.87 | 6.13 ± 1..6 / 6.37 ± 0.91 | 8.32 ± 0.87 /7.38 ± 1.11 | 7.24 ± 1.15 /6.58 ± 0.97 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Maximum dose | 59.53/ 55.69 | 48.39/ 52.17 | 50.31/53.27 | 49.27/48.02 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Minimum dose | 4.13/ 4.00 | 2.95/ 2.61 | 3.78/3.34 | 3.11/2.89 | > 0.005 | > 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| V25Gy (%) | 22.56 ± 2.37/19.27 ± 1.38 | 18.13 ± 0.97/ 18.75 ± 1.07 | 21.56 ± 1.63/ 19.29 ± 0.59 | 21.28 ± 1.19/19.99 ± 0.51 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| RCA | ||||||||||
| Mean dose | 3.35 ± 0.09 /2.81 ± 0.13 | 2.17 ± 0.24/ 2.46 ± 0.32 | 2.98 ± 0.18/ 2.64 ± 0.17 | 2.31 ± 0.14/ 2.17 ± 0..8 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Maximum dose | 45.61 / 38.63 | 41.39 / 38.19 | 43.54 / 40.27 | 42.05 / 39.21 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Minimum dose | 2.21/ 2.08 | 2.17/2.00 | 2.38/ 2.19 | 2.21/ 2.04 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| V25Gy (%) | 16.24 ± 1.21 / 13.26 ± 1.12 | 15.27 ± 1.35 / 12.36 ± 1.22 | 15.79 ± 1.53 / 12.98 ± 1.01 | 15.44 ± 0.86 / 12.51 ± 1.18 | > 0.005 | > 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Contralateral breast | ||||||||||
| Mean dose | 6.28 ± 0.12/ 5.27 ± 0.39 | 5.31 ± 0.77/ 4.51 ± 0.29 | 5.87 ± 0.61 / 5.17 ± 0.28 | 5.51 ± 0.31 / 5.03 ± 0.11 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Maximum dose | 8.37/7.21 | 7.07 /6.17 | 7.23 / 6.46 | 7.15 / 6.29 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| Minimum dose | 5.39/ 5.11 | 5.07/ 4.36 | 5.41/4.84 | 5.18/ 4.42 | < 0.005 | < 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| V20Gy (%) | 11.17 ± 0.94 /10.41 ± 0.39 | 9.75 ± 1.10 / 9.34 ± 0.56 | 10.15 ± 0.98 / 9.36 ± 0.88 | 9.91 ± 0.53/ 9.14 ± 0.27 | > 0.005 | > 0.005 | < 0.005 | > 0.005 | > 0.005 | > 0.005 |
| MHD | ||||||||||
| Mean (range) | 1.26 (0.9- 1.43) | 1.26 (0.9- 1.43) | 1.26 (0.9- 1.43) | 1.26 (0.9- 1.43) | - | - | - | - | - | - |
Abbreviations: BCS, Breast conserving surgery; 3D-CRT, 3-dimensional conformal radiotherapy; IMRT, Intensity modulated radiotherapy; RCA, right coronary artery; LAD, left ascending coronary artery
| 3D-Crtfor BCS-Post Mastectomy a | 6FIMRT Inverse Planning for BCS-Post Mastectomy a | 9FIMRT Inverse Planning for BCS-Post Mastectomy a | Hypofractionated IMRT for BCS-Post Mastectomy a | |||||
|---|---|---|---|---|---|---|---|---|
| MATLAB | R Environment | MATLAB | R Environment | MATLAB | R Environment | MATLAB | Renvironment | |
| TCP (%) | ||||||||
| Chest wall | 87/86 - 89/88 | 86/85 - 88/86 | 91/90 - 92/91 | 91/90 - 92/91 | 92/90 - 93/91 | 92/90 - 93/91 | 92/90 - 93/91 | 92/90 - 93/91 |
| Axilla | 90/90 - 90/90 | 91/91 - 91/91 | 92/92 - 93/93 | 92/92 - 93/93 | 92/92 - 93/93 | 93/92 - 94/93 | 92/92 - 93/91 | 93/92 - 94/92 |
| Supra-clavicu lar nodes | 94/94 - 96/96 | 94/94 - 94/93 | 94/94 - 95/94 | 94/94 - 95/94 | 94/94 - 95/95 | 94/94 - 95/95 | 94/94 - 95/95 | 94/93 - 95/94 |
| Internal mammary nodes | 93/86 - 97/90 | 93/85 - 97/89 | 95/89 - 97/91 | 96/90 - 97/91 | 96/91 - 97/91 | 96/90 - 97/91 | 96/91 - 97/91 | 96/90 - 97/91 |
| NTCP (%) | ||||||||
| Ipsilateral lung | 8/6 - 9/7 | 8/6 – 9/7 | 5/4 - 8/7 | 5/4 - 8/7 | 6/5 - 7/6 | 6/5 - 7/6 | 6/5 - 7/6 | 6/5 - 7/6 |
| Heart | 4/3 - 7/6 | 5/3 - 8/6 | 2/1 - 5/4 | 2/1 - 5/4 | 3/2 - 6/5 | 2/1 - 5/4 | 3/2 - 6/5 | 2/1 - 5/4 |
| LAD | 6/5 - 8/6 | 6/5 - 8/6 | 3/2 - 5/3 | 3/2 - 5/3 | 4/3 - 7/6 | 4/3 - 7/6 | 4/34/3 - 7/6 | 4/3 - 7/6 |
| RCA | 1/0.7 - 2/1.2 | 2/1.5 - 2.5/18 | 0.8/0.4 - 1.07/0.9 | 0.7/0.5 - 1.00/ 0.8 | 2/1.3 - 2/1.1 | 2/1.4 - 2/ 1.00 | 2/1.3 - 2/0.9 | 2/1.4 - 2/1.00 |
| Contralateral breast | 0.9/0.5 - 1.2/0.7 | 1.1/0.6 - 1.3 - 0.7 | 0.6/0.3 - 1.00/0.5 | 0.5/0.2 - 0.8/0.4 | 1.8/1.1 - 2/1.3 | 1.7/1.1 - 1.9/1.4 | 1.8/1.1 - 2/ 1.5 | 1.6/1.1 - 1.8/1.5 |
Abbreviations: TCP, tumor control probability; NTCP, normal tissue complications probability; BCS, breast conserving surgery; 3D-CRT, 3-dimensional conformal radiotherapy; IMRT, intensity modulated radiotherapy; RCA, right coronary artery; LAD, left ascending coronary artery
a With/without internal mammary fields
The mean (SD) imposed radiation doses (with/without internal mammary fields) to Ipsilateral lung, heart, LAD, RCA and contralateral breast of BCS patients (12.62 ± 0.56 / 12.21 ± 0.43, 4.95 ± 0.86 / 2.26 ± 0.72, 6.79 ± 1.25 / 4.12 ± 0.87, 1.17 ± 0.06 / 0.73 ± 0.18 and 3.36 ± 0.42 / 2.21 ± 0.21, respectively) were lower than post-mastectomy patients (15.37 ± 0.31 / 15.11 ± 0.34, 7.95 ± 0.28 / 5.64 ± 0.54, 9.83 ± 1.25 / 7.25 ± 0.87, 3.35 ± 0.09 / 2.81 ± 0.13, 6.28 ± 0.12 / 5.27 ± 0.39, respectively) for 3D-CRT (Tables 2 and 3). In addition, the V20Gy for Ipsilateral lung, and V25Gy for heart, LAD, RCA, and contralateral breast (with/ without internal mammary fields) of the BCS group were lower than the post-mastectomy group for the 3D- CRT method (Tables 2 and 3). The obtained TCP from MATLAB and R programs of 3D-CRT modality for the target volume including the chest wall surface, 3 levels of axilla, supraclavicular and internal mammary lymph nodes (with/ without internal mammary fields) for BCS patients were lower than post-mastectomy patients (Table 4), but it would seem that they were not significantly different. Moreover, for 3D-CRT, the generated NTCP (with/without internal mammary fields) from MATLAB and R programs for Ipsilateral lung, heart, LAD, RCA, and contralateral breast of the BCS group were lower than the post-mastectomy group (Table 4).
For the 6FIMRT, the mean (SD) imposed dose (with/without internal mammary fields) to Ipsilateral lung, heart, LAD, RCA and contralateral breast for BCS patients (8.32 ± 1.03 / 8.04 ± 0.87, 2.53 ± 1.18 /2.36 ± 0.69, 3.14 ± 1.37 / 3.93 ± 0.95, 0.71 ± 0.23 / 0.58 ± 0.27 and 2.12 ± 0.87 / 1.94 ± 0.23, respectively) were lower than mastectomy patients (11.74 ± 1.08 / 11.04 ± 0.34, 5.32 ± 1.18 /5.14 ± 0.69, 6.13 ± 1.6 / 6.37 ± 0.91, 2.17 ± 0.24/ 2.46 ± 0.32 and5.31 ± 0.77 / 4.51 ± 0.29, respectively). Furthermore, for the Ipsilateral lung, the V20Gy and the V25Gy for heart, LAD, RCA contralateral breast (with/without internal mammary fields) of the post-mastectomy RT group were higher than BCS patients (Tables 2 and 3). Table 4 indicates that for the 6FIMRT approach, TCP of the stated targets (with/without internal mammary fields) for BCS was lower than the mastectomy group. Also, for the 6FIMRT, the NTCP of the Ipsilateral lung, heart, LAD, RCA contralateral breast of BCS were lower compared to the mastectomy group.
For the 9FIMRT, the mean (SD) imposed doses (with/without internal mammary fields) to ipsilateral lung, heart, LAD, RCA, and contralateral breast of BCS patients (10.57 ± 1.01 / 9.91 ± 0.74, 3.62 ± 1.18 / 4.51 ± 0.84, 5.46 ± 1.83 / 4.38 ± 1.04, 0.92 ± 0.14 / 0.89 ± 0.11 and 2.57 ± 0.34 / 2.07 ± 0.14, respectively) were lower than post-mastectomy RT females (13.41 ± 1.18 / 12.39 ± 0.63, 6.28 ± 1.09 / 6.11 ± 0.73, 8.32 ± 0.87 / 7.38 ± 1.11,2.98 ± 0.18 / 2.64 ± 0.17 and 5.87 ± 0.61 / 5.17 ± 0.28, respectively). In addition, for the ipsilateral lung, the V20Gy and the V25Gy for heart, LAD, RCA, and contralateral breast of BCS patients (with/without internal mammary fields) were lower than other groups. Table 4 indicates that TCP of the stated target volumes for BCS patients was lower than mastectomy patients (with/without internal mammary fields) for the 9FIMRT method. Nevertheless, the NTCP of the Ipsilateral lung, heart, LAD, RCA, and contralateral breast for mastectomy patients was higher than BCS patients.
Tables 2 and 3 also show some data about hypofractionated IMRT. As can be seen, the mean (SD) imposed dose (with/without internal mammary fields) to ipsilateral lung, heart, LAD, RCA and contralateral breast for BCS patients (10.23 ± 1.00 / 9.81 ± 0.78, 3.37 ± 1.07 / 4.26 ± 0.84, 5.24 ± 1.15 /4.24 ± 0.97, 0.89 ± 0.10/ 0.82 ± 0.11 and 2.41 ± 0.31 / 2.03 ± 0.11, respectively) were lower than mastectomy patients (12.24 ± 1.12 / 11.41 ± 0.39, 6.10 ± 0.99 / 6.07 ± 0.57, 7.24 ± 1.15 /6.58 ± 0.97 and 5.51 ± 0.31 / 5.03 ± 0.11, respectively). Moreover, for the hypofractionated method, the V20Gy for ipsilateral lung and V25Gy for heart, LAD, RCA, and contralateral breast of BCS patients were lower in comparison with mastectomy patients. Table 4 illustrates that the TCPs of the mentioned target volumes for the hypofractionated modality of mastectomy patients were higher than BCS patients. Nonetheless, the NTCPs of OARs for the BCS patients were lower than mastectomy patients.
5. Discussion
For patients with breast cancer, tangential plans are considered the most common method for RT of BCS and mastectomy patients. Several studies have discussed the imposed radiation dose to OARs such as ipsilateral lung, heart, and LAD (17-20). However, to the best of our knowledge, there is limited data about the imposed radiation dose to other organs such as LAD and RCA for 3D-CRT, IMRT, and hypofractionated modalities for the discussed patients. In addition, it would seem that data about the evaluation of TCP and NTCP models using MATLAB and R programs for the target volumes and OARs among the mentioned method for BCS and mastectomy patients are scarce. Therefore, this study aimed at evaluating the imposed dose and TCP-NTCP models for the target volumes and OARs including ipsilateral lung, heart, LAD, and RCA using MATLAB and R programs with and without internal mammary nodes.
Based on our findings, the mean (± SD) imposed dose (with/without internal mammary fields) to ipsilateral lung, heart, LAD, and RCA for 3D-CRT were significantly higher compared to 6 and 9 fields IMRT inverse plans (P < 0.005) for both BCS and post-mastectomy patients. Tables 2 and 3 illustrate that the mean (± SD) imposed radiation dose to the studied OARs for 6FIMRT was significantly lower than 3D-CRT (P < 0.005). In addition, the mean (± SD) dose of 6FIMRT for the discussed OARs was lower compared to 9FIMRT and hypofractionated IMRT, but it was not significant (P > 0.005). Furthermore, the V20Gy for Ipsilateral lung and the V25Gy for heart, LAD, and RCA of 6 and 9 fields and hypofractionated (with/without internal mammary fields) were significantly lower compared to 3D-CRT (P < 0.005) for both groups, while there were no significant differences for the V20Gy of ipsilateral lung and the V25Gy of heart, LAD, and RCA among 6 and 9 fields with hypofractionated RT (P > 0.005). Moreover, it was found that the mean (± SD) imposed dose to LAD was higher compared to heart and RCA for all studied modalities, while the imposed dose to RCA was lower than heart and LAD among the mentioned RT methods for BCS and post-mastectomy patients (Tables 2 and 3), which is highly due to their different anatomical features. Furthermore, it is considered that the minimum dose of heart, LAD, and RCA were not significantly different for the 3D-CRT, IMRT, and hypofractionated RT (P > 0.005).
Our findings are in an agreement with the study of Taylor et al. who stated that the imposed RT dose to the anterior part of the heart including LAD is higher than the whole heart (30). Recently, Gocer and Ozer have performed a dosimetric study on OARs of patients with breast cancer including heart, LAD, left circumflex coronary artery, right and left ventricles, using tangential beams of 3D-CRT. In their study, they concluded that the mean radiation dose to LAD was higher than the heart and its coronary arteries for patients with left-sided breast cancer (20), which is in line with our study. In addition, in their study, they have reported that the highest maximum radiation dose was for the heart for patients with left-sided breast cancer (20). Moreover, the results of our study are similar to Rudat et al. who mentioned that the tangential IMRT plans may significantly reduce the dose-volumes of the ipsilateral lung and heart compared to tangential 3D-CRT for post-mastectomy breast cancer patients (10). Aznar et al. have focused on the imposed dose to heart, arch, and also whole LAD in respiration-adapted RT of left-sided breast cancers. In this study, they found that the radiation dose to arch and whole LAD was different for some of their patients, and, thus, it is demonstrated that the assessment of the dose to the whole heart as well as to the whole LAD is crucial for the stated patients (17).
Based on Table 2, the TCP of the stated target volumes (with/without internal mammary fields) for 9FIMRT and hypofractionated RT were higher than 6 fields and 3D-CRT, but they were not significant (P > 0.005). Whereas, the NTCP of the Ipsilateral lung for 6FIMRT was lower compared to the 9 fields and hypofractionated RT (P > 0.005). Also, the NTCP for 3D-CRT was significantly higher than other methods (P < 0.005). Li et al. have found that the TCP of conventional RT and IMRT is more than 90%, but the NTCP of the lung for IMRT is less than the conventional method (31), which is similar to our results. Furthermore, our findings showed that the NTCP of the heart and its coronary arteries including LAD and RCA for 6FIMRT was lower compared to 3D-CRT, 9FIMRT, and hypofractionated methods. Moreover, our data showed that the hypofractionated RT may decrease the treatment time, which is in line with Li et al. who mentioned that their hypofractionated technique may reduce the RT time for their patients with breast cancer (31).
According to the results of our study, 6FIMRT not only may provide suitable PTV dose coverage but also may impose lower complication probabilities to OARs compared to 9FIMRT, hypofractionated RT, and 3D-CRT. Nevertheless, the hypofractionated method may be a good alternative to reduce breast cancer treatment time.
5.1. Conclusions
In this study, the dose distribution and TCP-NTCP models of 3D-CRT, 6 and 9 fields IMRT inverse plans, and hypofractionated IMRT are evaluated for patients with left-sided breast cancer (BCS and mastectomy). Based on our findings, 6FIMRT inverse planning is well worth treating patients with left-sided breast cancer due to enough dose coverage for PTV, suitable TCP for target volumes, and lower NTCP for ipsilateral lung, heart, LAD, and also RCA compared to others. However, as a result of lower treatment time for the hypofractionated IMRT, using this modality is suggested.
According to our findings of the above-discussed methods, further research about the advantages and disadvantages of hypofractionated RT for BCS and post-mastectomy patients is proposed.