1. Background
Thyroid nodules are lesions in the thyroid that are different from the surrounding parenchymal tissue. Thyroid nodules prevalence in ultrasound examinations has been estimated at 60%. The malignancy rate in thyroid nodules is 5 to 10% and most thyroid nodules are benign. Thyroid cancer is the most common endocrine-related malignancy, accounting for 1.3% of all cancers worldwide. Papillary thyroid cancer (PTC) is the most common type of thyroid cancer and accounts for more than 80% of thyroid cancers. An increase in the incidence of thyroid cancer is reported in recent years (1-4). Apart from thyroid ultrasonography and ultrasound-guided fine-needle aspiration biopsy in the early stages of cancer, which have led to an increase in the diagnosis of small thyroid nodules, the role of other unidentified factors in the increased incidence of these malignancies requires further investigations (5, 6).
Currently, the most well-known thyroid cancer risk factors include the history of cancer in first-degree relatives, head and neck radiation in childhood (7), and insufficient or excessive intake of iodine (8). But the recent rise in the incidence of thyroid cancer is not justified by any of these factors. Different risk factors such as metabolic syndrome, diabetes, obesity (9), insulin resistance (10), chemical toxins (11), nutritional factors (12), and infections have been recently suggested as potential risk factors for thyroid cancer. Regarding benign thyroid nodules, risk factors including insulin resistance (13), and infectious agents, such as Helicobacter pylori (14), have been introduced in recent years.
Helicobacter pylori is a microaerophilic, helical-shaped, and Gram-negative bacterium, which colonizes the mucosa of the stomach. This bacterium is involved in gastric diseases, such as peptic ulcers, gastritis, and stomach cancer (15-17). Infection with H. pylori leads to severe infiltration of polymorphonuclear cells into the mucosa of the stomach. If the infection is not effectively cleared, this acute infiltration gradually leads to a chronic infiltration of mononuclear cells through the immune system. On the other hand, chronic infiltration of the mononuclear cells results in the production of local pro-inflammatory cytokines, whose systemic diffusion will affect distant tissues and various systemic organs. Accordingly, H. pylori infection has been associated with some diseases outside the gastrointestinal tract (18, 19).
Some studies have shown an association between H. pylori infection and autoimmune thyroid disease (20-23), but to our knowledge, only one study has examined the association between H. pylori infection and benign nodules. In recent years, insulin resistance has been introduced as one of the mechanisms involved in nodule formation. Helicobacter pylori infection may play a pathogenic role in the evolution of insulin resistance (14, 24).
2. Objectives
Concerning limited data in this field, we conducted this case-control study to investigate the association between H. pylori infection and the presence of benign and malignant thyroid nodules separately in comparison to a healthy euthyroid control group without nodules; in an Iodine sufficient area.
3. Methods
This case-control study was performed between March 2018 and October 2020 on euthyroid patients with thyroid nodules who were referred to endocrinology clinics in Zahedan, southeastern Iran.
Inclusion criteria were: age ≥ 18 years, presence of thyroid ≥ 1 cm, and normal thyroid function tests (TSH: 0.4 - 4.2 mIU/L, FT4: 0.8 - 1.8 ng/dL, and FT3: 2.3 - 4.2 pg/mL) (25). Subjects with previous head and neck radiation, surgery for thyroid disease, thyroid dysfunction, thyroid medications, receiving contrast for imaging during the last 6 months, diabetes mellitus, liver failure, and kidney failure were excluded. Also, women who were pregnant or lactating were not included in the study.
All participants underwent thyroid sonography that was performed by a sonologist. If a nodule larger than or equal to one centimeter was reported on ultrasound, the nodule was examined by fine-needle aspiration biopsy. The cytology was reported, based on the Bethesda system (26). Patients suspected of papillary thyroid cancer based on the cytology result underwent total thyroidectomy. These patients were included in the PTC group if the permanent pathology confirmed the diagnosis of PTC. Patients with benign cytology report were classified as the benign thyroid nodule group. Non-first-degree relatives of the patients and hospital staff without thyroid disease were chosen as the control group after considering the inclusion and exclusion criteria. Patients in the control group underwent thyroid ultrasound. If no nodules with any size were reported on their ultrasound, they were included in the control group. They were apparently healthy and had no evidence of any acute or chronic illness on history or physical examination. Socio-economic level and geographical area of the control group were similar to the case group. Finally, three groups; including the PTC group, benign thyroid nodule group, and control group were recruited for this study.
Height using a stadiometer and weight with minimal clothing using a digital scale was measured. Blood samples were recruited from all subjects. Samples were stored at -70°C until the day of testing. Thyroid function tests and H. pylori Immunoglobulin (Ig) G were measured. TSH, FT3, and FT4, by an automated analyzer using immunochemoluminescent assays were measured. Helicobacter pylori IgG was assessed by the enzyme-linked immunoassay method. Values above 10 IU/mL were considered positive.
This study was approved by the Zahedan University Ethics Committee for Human Studies (ethical code number: IR.ZAUMS.REC.1399.091). The participants provided written, informed consent.
3.1. Statistical Analysis
Continuous variables were presented as mean and standard deviation (SD) and categorical variables as absolute number and percentages. One-way ANOVA test was used to compare a numerical variable in three study groups. An independent t-test or a Mann–Whitney U-test was used to assess significance of differences for continuous variables and a chi-square or Fisher's exact test for categorical variables. Bonferroni correction for continuous variables and chi-square test for categorical variables was used for a Post-hoc pairwise comparison of these three study groups. The correlation between H. pylori antibody (IgG) titers with numerical variables was assessed with the Pearson correlation coefficient, and an important correlation was presented in scatter plots with quadratic fitting and a 95% confidence interval. P-value lower than 0.05 was considered statistically significant. All of the analyses were conducted with Stata statistical software: Release 14. College Station, TX: StataCorp LP.
4. Results
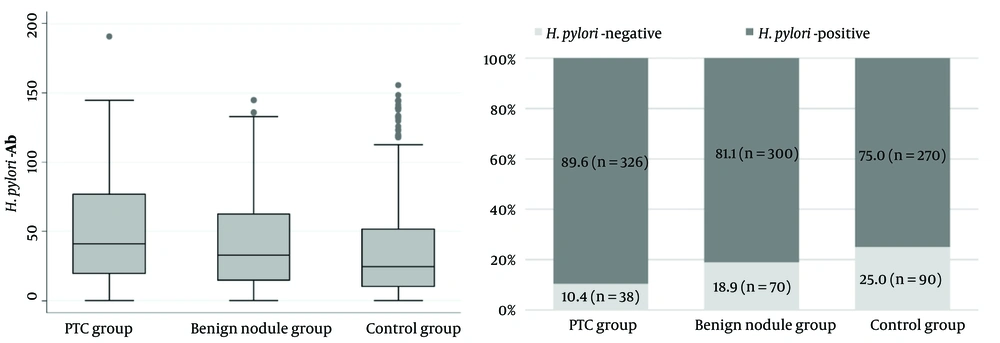
Baseline characteristics of study participants are presented in Table 1. There was no statistically significant difference in age, sex, and BMI in these groups. Although all participants were euthyroid, levels of TSH were higher in patients with PTC than in patients with benign thyroid nodules and controls. The prevalence of H. pylori infection in PTC patients was 89.6% which was significantly higher than the groups of benign thyroid nodules (81.1%) and the control group (75.0%) as shown in Figure 1.
| Variables | Patients with PTC (n = 364) | Patients with Benign Nodule (n = 370) | Control Subjects (n = 360) | P-Value |
|---|---|---|---|---|
| Age (y) | 34.95 ± 11.94 | 34.99 ± 12.81 | 34.63 ± 12.98 | 0.855 |
| Sex (female) | 290 (79.7) | 296 (80.0) | 290 (80.6) | 0.956 |
| Body mass index (Kg/m2) | 24.61 ± 3.88 | 24.91 ± 3.66 | 24.21 ± 4.31 | 0.609 |
| Thyroid stimulating hormone (mIu/L) | 2.49 ± 1.11 A | 1.79 ± 1.03 B | 2.10±1.01 C | < 0.001 |
| Free T4 (ng/dL) | 1.29 ± 0.30 | 1.30 ± 0.20 | 1.31 ± 0.25 | 0.406 |
| Free T3 (pg/mL) | 3.49 ± 0.59 | 3.60 ± 0.66 | 3.58 ± 0.59 | 0.104 |
| Helicobacterpylori-Ab titer (Iu/mL) | 49.62 ± 35.75 A | 42.74 ± 36.54 B | 37.22 ± 36.84 B | < 0.001 |
| Positive H. pylori | 326 (89.6) A | 300 (81.1) B | 270 (75.0) B | < 0.001 |
aData are expressed as mean ± SD or No. (%).
bPost-Hoc pairwise comparison based-on Bonferroni method for continuous variables and chi-square test for categorical variables. A, B, C in the same row of variables shows significant (P < 0.05) difference between the means while same letters in one row shows non-significant difference between the means of three group.
In patients with PTC; Vascular Invasion in 5.5%, extrathyroid extension in 3.8%, Capsular invasion in 8.8%, and multifocality in 12.1% was observed. The mean of H. pylori antibody and also the prevalence of H. pylori infection in these groups were not significantly different. Although the prevalence of H. pylori infection in different cancer stages and tumor size categories was not significantly different, the mean of H. pylori antibody in lower stages of cancer, as well as small tumors, was lower (Table 2).
| No. (%) | Helicobacter pylori-Ab Titer | H. pylori (> 10) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | P-Value | Negative (%) | Positive (%) | P-Value | ||
| Multifocality | 0.350 | 0.173 | ||||
| Absent | 320 (87.91) | 48.97 ± 35.86 | 36 (11.3) | 284 (88.8) | ||
| Present | 44 (12.09) | 54.35 ± 34.96 | 2 (4.5) | 42 (95.5) | ||
| Extrathyroidal extension | 0.151 | 0.681 | ||||
| Absent | 350(96.15) | 49.08 ± 35.54 | 37 (10.6) | 313 (89.40 | ||
| Present | 14(3.85) | 63.08 ± 39.70 | 1 (7.1) | 13 (92.9) | ||
| Capsular invasion | 0.848 | 0.417 | ||||
| Absent | 332 (91.21) | 49.51 ± 35.77 | 36 (10.8) | 296 (89.2) | ||
| Present | 32 (8.79) | 50.78 ± 36.04 | 2 (6.3) | 30 (93.8) | ||
| Vascular invasion | 0.900 | 0.947 | ||||
| Absent | 344 (94.5) | 49.68 ± 35.90 | 36 (10.5) | 308 (89.5) | ||
| Present | 20 (5.5) | 48.65 ± 33.79 | 2 (10.0) | 18 (90.0) | ||
| Nodal involvement | 0.893 | 0.878 | ||||
| N0 | 308 (84.61) | 50.00 ± 35.49 | 32 (10.4) | 276 (89.6) | ||
| N1a | 42 (11.54) | 47.61 ± 38.13 | 5 (11.9) | 37 (88.1) | ||
| N1b | 14 (3.85) | 47.29 ± 36.55 | 1 (7.1) | 13 (92.9) | ||
| Cancer stage | 0.019 | 0.377 | ||||
| I | 320 (87.91) | 47.52 ± 34.72 | 37 (11.6) | 283 (88.4) | ||
| II | 22 (6.04) | 61.62 ± 29.42 | 0 (0.0) | 22 (100.0) | ||
| III | 8(2.20) | 82.98 ± 59.81 | 0 (0.0) | 8 (100.0) | ||
| IVa | 12 (3.30) | 59.30 ± 42.41 | 1 (8.3) | 11 (91.7) | ||
| IVb | 2 (0.55) | 61.78 ± 38.35 | 0 (0.0) | 2 (100.0) | ||
| Tumor size category | 0.109 | 0.410 | ||||
| T1a | 116 (31.9) | 44.43 ± 33.28 | 15 (12.9) | 101 (87.1) | ||
| T1b | 108 (29.7) | 50.35 ± 39.01 | 13 (12.0) | 95 (88.0) | ||
| T2 | 110 (30.2) | 53.16 ± 34.16 | 9 (8.2) | 101 (91.9) | ||
| T3 | 20 (5.5) | 43.17 ± 32.50 | 0 (0.0) | 20 (100) | ||
| T4a | 6 (1.6) | 65.26 ± 40.86 | 1 (16.7) | 5 (83.3) | ||
| T4b | 4 (1.1) | 82.56 ± 46.89 | 0 (0.0) | 4 (100) | ||
| Tumor size: T1 vs >T1 | 0.115 | 0.104 | ||||
| T1 | 112 () | 53.36 ± 34.82 | 10 (7.1) | 130 (92.9) | ||
| >T1 | 70 () | 47.28 ± 36.20 | 28 (12.5) | 196 (87.5) | ||
| Histological type | 0.258 | 0.247 | ||||
| Classic | 306 (84.07) | 50.68 ± 35.56 | 29 (9.5) | 277 (90.5) | ||
| Follicular | 50 (13.74) | 45.93 ± 37.18 | 7 (14.0) | 43 (86.0) | ||
| Tall cell | 8 (2.20) | 32.13 ± 31.75 | 2 (25.0) | 6 (75.0) | ||
Clinical features and biochemical characteristics comparison of H. pylori positive and negative patients in three study groups are shown in Table 3. None of these clinical and laboratory characteristics were significantly different in any of the groups.
| Patients with PTC (n = 364) | Patients with Benign Nodule (n = 370) | Control Group (n = 360) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative H. pylori | Positive H. pylori | P-Value | Negative H. pylori | Positive H. pylori | P-Value | Negative H. pylori | Positive H. pylori | P-Value | |
| Sex | 0.757 | 0.740 | 0.645 | ||||||
| Female | 31 (81.6) | 259 (79.4) | 55 (78.6) | 241 (80.3) | 74 (82.2) | 216 (80.0) | |||
| Male | 7 (18.4) | 67 (20.6) | 15 (21.4) | 59 (19.7) | 16 (17.8) | 54 (20.0) | |||
| Age (y) | 35.61 ± 13.10 | 35.43 ± 11.64 | 0.938 | 34.44 ± 12.41 | 35.20 ± 13.09 | 0.649 | 36.53 ± 13.45 | 34.43 ± 12.88 | 0.196 |
| Body mass index (Kg/m2) | 24.32 ± 3.74 | 24.59 ± 3.92 | 0.676 | 24.58 ± 4.76 | 24.23 ± 3.75 | 0.508 | 23.50 ± 3.88 | 24.60 ± 4.14 | 0.027 |
| Free T4 (ng/dL) | 1.33 ± 0.23 | 1.29 ± 0.25 | 0.397 | 1.27 ± 0.22 | 1.29 ± 0.25 | 0.491 | 1.33 ± 0.24 | 1.30 ± 0.24 | 0.385 |
| Free T3 (pg/mL) | 3.60 ± 0.53 | 3.58 ± 0.61 | 0.876 | 3.66 ± 0.60 | 3.68 ± 0.70 | 0.787 | 3.59 ± 0.43 | 3.62 ± 0.62 | 0.729 |
| Thyroid stimulating hormone (mIu/L) | 2.38 ± 1.13 | 2.53 ± 1.05 | 0.399 | 1.81 ± 1.00 | 1.89 ± 1.00 | 0.531 | 2.03 ± 0.92 | 2.07 ± 1.11 | 0.705 |
aData are expressed as mean ± SD or No. (%).
The correlation between H. pylori antibody titer and anthropometric, biochemical characteristics in study groups are shown in Table 4. Helicobacter pylori antibody was not significantly correlated with any of the anthropometric and biochemical variables.
| H. pylori-Ab Titer | |||
|---|---|---|---|
| PTC Group | Benign Nodule Group | Control Group | |
| Age | |||
| r | -0.008 | 0.051 | -0.054 |
| P-value | 0.886 | 0.327 | 0.303 |
| BMI | |||
| r | -0.076 | 0.003 | -0.005 |
| P-value | 0.146 | 0.954 | 0.924 |
| FT4 | |||
| r | -0.006 | 0.062 | -0.006 |
| P-value | 0.903 | 0.231 | 0.913 |
| FT3 | |||
| r | -0.037 | -0.011 | -0.105* |
| P-value | 0.481 | 0.835 | 0.047 |
| TSH | |||
| r | -0.005 | -0.029 | -0.069 |
| P-value | 0.925 | 0.575 | 0.191 |
| Tumor size | |||
| r | 0.029 | -0.060 | |
| P-value | 0.578 | 0.247 | |
5. Discussion
The present study showed a significant association between H. pylori infection and benign or malignant thyroid nodules. The prevalence of H. pylori infection was estimated at 89% in patients with PTC, 81% in patients with benign thyroid nodules, and 75% in the euthyroid control group without nodules. Also, the rate of infection was significantly different between the benign and malignant thyroid nodules. These findings are consistent with a study conducted in China, which demonstrated an association between H. pylori infection and thyroid nodules (14). To our knowledge, no study has been conducted on the relationship between H. pylori infection and thyroid cancer.
Thyroid nodules are characterized by the overgrowth of one or more areas in the thyroid gland. The etiology of thyroid nodules involves interactions between genetic and environmental factors (27). Although factors such as increasing the use of imaging techniques and more rapid detection of small thyroid nodules have been implicated in the recent increase in the incidence of thyroid cancer, but some environmental factors such as infectious agents may be involved (28-30)
Recently, an association has been found between infectious agents and thyroid nodules (14, 31-33). Helicobacter pylori infection is an environmental risk factor, which may mimic the antigenic profile of thyrocyte membranes and can have a considerable role in autoimmune thyroid disease pathogenesis (20-23). Helicobacter pylori is a Gram-negative labyrinthos bacterium, which can be mainly found in the mucous membrane and is responsible for the most common bacterial infection in humans (34). In Iran, the prevalence of H. pylori infection ranges from 36% to 90% in different geographical areas (35). In the present study, despite the high prevalence of this infection in the study population, an association was found between thyroid nodules and this infection. However, the mechanism responsible for this association has not been determined yet.
Thyroid-stimulating hormone (TSH) plays a key role in regulating the growth and differentiation of thyroid cells and acts as a mitogen in cell cultures by reducing apoptosis (35). In the present study, TSH was in the normal range, therefore, the present results cannot be justified by TSH.
Moreover, the insulin-like growth factor (IGF) is an important growth and differentiation factor in different cell types. The IGF system consists of a network of ligands, including IGF-I, IGF-II, and their receptors, with significant homology to insulin and insulin receptors (36). Insulin/IGF-1 signaling pathway is involved in regulating thyroid gene expression, differentiation and proliferation of thyroid cells (37). Previous studies have shown that IGF-1, IGF-2, IGF-1 receptors, and insulin receptor isoforms are abundant in thyroid follicular cell precursors. Therefore, activation of the IGF system and the insulin pathway may explain the present findings. Insulin resistance and abnormal glucose metabolism increase insulin levels, which in turn augment thyroid cell proliferation and thyroid nodule formation. In recent years, insulin resistance has been introduced as one of the mechanisms involved in nodule formation (10, 38). Helicobacter pylori infection may play a pathogenic role in the evolution of insulin resistance (39). Therefore, activation of the insulin pathway may explain the present results, although further investigation is required in this area.
On the contray, previous researches have shown an association between vitamin D and thyroid cancer (39, 40). These studies have shown that vitamin D exerts its anti-cancer effects by increasing apoptosis, ending the cell cycle, increasing differentiation, inhibiting proliferation, and reducing invasion (41, 42).
Molecular modeling has revealed that a type of bacterium produces a substance, which can inactivate vitamin D receptors, resulting in the decreased level of 25-hydroxyvitamin D receptors and the increased level of 1, 25-dihydroxy vitamin D (43). It has been shown that 1, 25-dihydroxy vitamin D has a strong tendency to bind to α- thyroid receptors. If the transcription of α- thyroid receptor is impaired, numerous metabolic dysfunctions will occur (44). However, it is unclear whether this mechanism can be generalized to H. pylori infection.
This study had several limitations. First, it had a cross-sectional design, which could not show the cause-and-effect relationship between H. pylori infection and thyroid nodules. Second, to identify infection, we used IgG antibodies against H. pylori, which could not differentiate between acute and chronic infections. The main strengths of this study were including a nodule-free euthyroid control group, cytological evaluation of nodules, histopathological examination of thyroid nodule outcomes, and adequate sample size.
5.1. Conclusions
In summary, our results showed that the rate of H. pylori infection was higher in patients with malignant and benign thyroid nodules compared to the control group. Further studies are needed to establish such an association and to identify the mechanisms that cause it to take steps to prevent the occurrence of such nodules.