1. Background
Triple-negative breast cancer (TNBC) is the most lethal subtype of breast cancer and the hardest type to cure (1). Metastasis vastly increases the fatality of cancer (2, 3). Tumor cells need to degrade the extracellular matrix (ECM) protein to successfully metastasize (4). It has been shown that the MMP-2 and -9 enzymes play a major role in cancer cell metastasis through ECM degradation (5, 6).
As the most common way to confront cancer, chemotherapy has various adverse effects on normal organs (7). Therefore, researchers consider using natural compounds with anticancer effects as an alternative or additional therapies for cancer (8, 9).
Doxorubicin (dox) is a glycoside antibiotic that is widely used for breast cancer treatment (10). It acts through various mechanisms that lead to the impairment of DNA replication (11, 12). However, the exact molecular mechanisms of dox that affect the expression of different genes are not clear yet. It has been observed that dox shows synergistic effects with natural compounds in various cancers (13, 14).
Quercetin (que) is a flavonol that is found in various plants. The anticancer effects of que are mostly related to its anti-inflammatory capabilities. The results of previous studies show that que inhibits the activity of significant signaling pathways such as PI3K-AKT and ERK pathways, which have regulating role in the expression of MMP enzymes. This leads to downregulation of several genes, including MMP-2 and -9 through inhibition of the NF-kB pathway as a downstream of PI3K-AKT and ERK pathways (15, 16). It has been observed that que synergizes with some chemotherapeutic agents in different types of cancer (17, 18). Nevertheless, the effects of que in combination with dox on metastatic breast cancer cells’ migration are not yet clear.
2. Objectives
This study investigates the effects of que alone and combined with dox on the migration of the MDA-MB-231 breast cancer cell line and the expression of MMP-2 and MMP-9 genes in vitro.
3. Methods
3.1. Cell Culture and Reagents
TNBC cell line MDA-MB-231 and normal human lung fibroblast cell line MRC5, which grow properly in low glucose (4.5 g/L) DMEM, 10% FBS, and 1% penicillin/streptomycin antibiotics (purchased from Bio-Idea Tehran, Iran) and in an incubator with 5% CO2 and 37°C, were purchased from the Pasteur Institute (Tehran, Iran).
3.2. Treatments
Dox (Ebewe pharma, Unteracht, Austria) with 2 mg/mL concentration was directly diluted in medium to obtain the desired concentrations. Que (Sigma-Aldrich, St. Louis, Missouri, United States) was prepared before treatment through dilution in DMSO (Bio-Idea) and was, then, diluted in the culture medium. The concentration of DMSO was constantly maintained at < 0.1% so as not to affect the viability of the cells (19).
3.3. Cell Viability Assay (MTT)
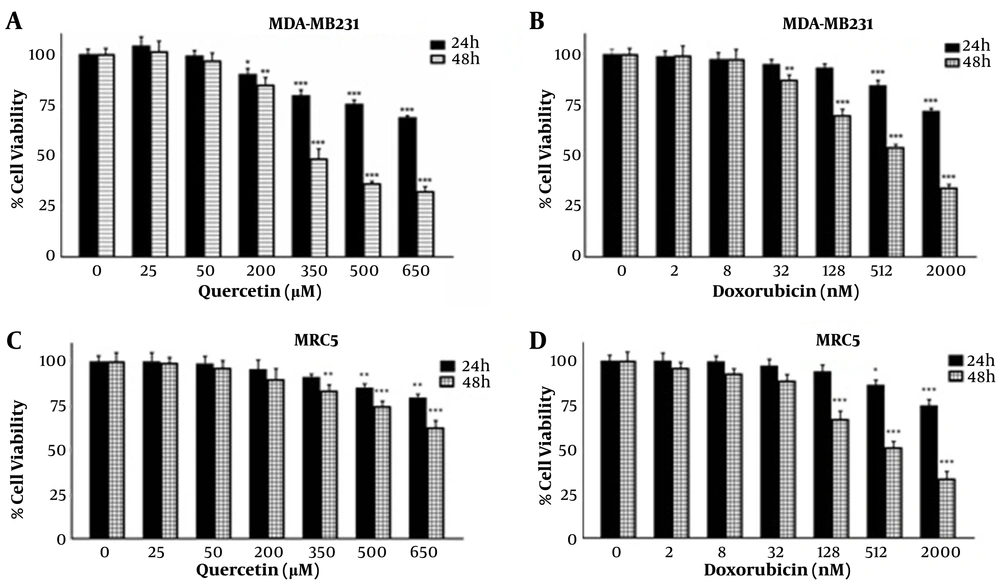
Cell viability was assessed by MTT assay. MDA-MB-231 (4 × 103 cells per well) and MRC5 (104 cells per well) cells in 96-well plates were treated with que (25, 50, 200, 350, 500, and 650 μM) and dox (2, 8, 32, 128, 512, and 2000 nM) for either 24 or 48 hours. Optical density at 570 nM was read for each well using a microplate reader (BioTek ELx800 Winooski, Vermont, United States). The IC50 value was obtained, using Curve Expert 1.3. SI was calculated for drugs, using the following formula: SI = IC50 of normal cells/IC50 of cancer cells. The SI values > 1 show the drug has more effects on cancer cells compared to normal ones (20, 21).
3.4. CI and DRI Calculation
Drug synergism was evaluated, using the Combination Index (CI) value. CI > 1, CI < 1, and CI = 1 represent antagonism, synergy, and additive effects, respectively. Dose reduction index (DRI) value was calculated, using CompuSyn 1.0 software (Chou and Martin, 2005, CompuSyn Inc, USA) to determine the magnitude of drug dose reductions (22).
3.5. Wound Healing Assay
Cancer cells (106) with about 80% confluency in 6-well plates were wounded, using a sterile 200 μL pipette tip and, then, washed with PBS to remove detached cells. The cells were supplemented with the medium containing drugs and 4% FBS and, then, incubated for 48 h. The wounds were photographed at 0, 24, and 48 h. The gap areas were measured, using Image J software (National Institutes of Health, Bethesda, USA). Migration rate was calculated, using the following formula:
Migration rate = [(T0 – Th)/T0] × 100 (23)
3.6. Total RNA Extraction and cDNA Synthesis
A total of 106 cells were used for RNA extraction, using a Hybrid-R RNA extraction kit (GeneAll, Songpa-gu, Seoul, South Korea). RNA purity and integrity were evaluated, using A260/A280 ratio and agarose gel electrophoresis, respectively. The cDNA synthesis kit (Yekta Tajhiz Azma, Tehran, Iran) with a 20 μL mixture solution was used for cDNA synthesis.
3.7. Real-time qPCR
MMP-2 and -9 genes expression were evaluated by Real-time qPCR, using SYBR green kit (Yekta Tajhiz Azma, Tehran, Iran) with the following primers:
(1) MMP-2
F: 5’-CCCAGCCAGAAGCGGAAA-3’
R: 5’- CGAACAGATGCCACAATAAAGC-3’
(2) MMP-9
F: 5’-CCTTTGGACACGCACGAC-3’
R: 5’-CCACCTGGTTCAACTCACTC-3’
(3) HPRT
F: 5’ GACCAGTCAACAGGGGACAT 3’
R: 5’ CCTGACCAAGGAAAGCAAAG 3’
The HPRT was selected as the internal reference gene. The amplified fragments length of MMP-2, MMP-9, and HPRT genes were 198, 103, and 132 base pairs, respectively. The reaction conditions were as follows: 95˚C for 3 min; 95˚C for 10 sec, 59˚C for 10 sec, 72˚C for 20 sec for 40 cycles; 72˚C for 5 min (23).
3.8. Statistical Analysis
All data were reported as mean ± SEM of 3 separate tests. SPSS 26.0 software (IBM, SPSS Inc.) was used for statistical analysis. The results of different experimental groups were compared to each other, using One-way ANOVA and LSD post-hoc tests with P-values less than 0.05 considered significant.
4. Results
4.1. Quercetin Enhances the Effect of Dox on Cancer Cell Viability
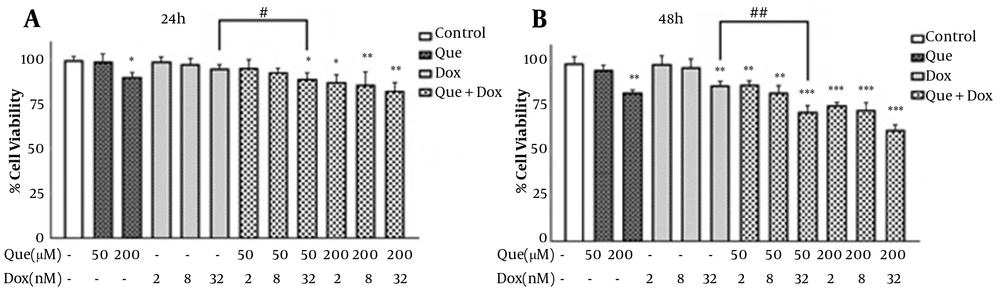
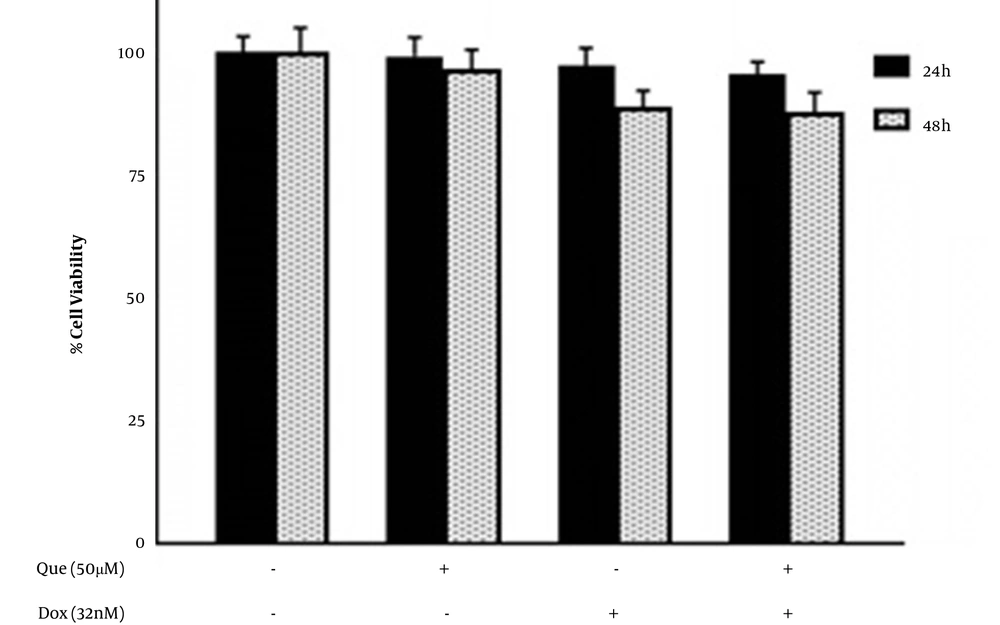
Cells were treated with various concentrations of que and dox for either 24 or 48 hours. Figures 1A and B show that que and dox inhibited the cancer cell viability significantly. Figures 1C and D show the effects of que and dox on normal cells, respectively. SI values represented in Table 1 show high inhibitory effects of que on cancer cells and higher adverse effects of dox on normal cells. According to Figure 2, all combination states significantly reduced the viability of cancer cells compared to each drug alone. As shown in Table 2, the CI values for all combination states were below 1, which indicates that que and dox synergize in all concentrations. We chose the combination state with the lowest CI (0.36) (50 μM of que and 32 nM of dox) for the rest of the study. The DRI value for this combination state was 5.7, which showed a 5-time reduction in dox dose (results not shown). Eventually, Figure 3 shows that the selected combination state had no significant effects on the normal cells.
The effects of quercetin and doxorubicin on the viability of MDA-MB-231 and MRC5 cell line through MTT assay. (A) The effects of different concentrations of quercetin on MDA-MB-231 cell viability in 24 and 48 h. (B) The effects of different concentrations of doxorubicin on MDA-MB-231 cell viability in 24 and 48 h. (C) The effects of different concentrations of quercetin on MRC5 cell viability in 24 and 48 h. (D) The effects of different concentrations of doxorubicin on MRC5 cell viability in 24 and 48 h. The results are presented as mean ± SEM of at least 3 independent experiments *P < 0.05; **P < 0.01; ***P < 0.001 significant from control untreated cells.
| Cell Line | IC50 | |
|---|---|---|
| Dox (nM) | Que (µM) | |
| MDA-MB-231 | 640 | 295 |
| MRC5 | 540 | > 1000 |
| SI | 0.84 | > 3.39 |
The effects of combinations of quercetin and doxorubicin on the viability of MDA-MB-231 cells through MTT assay. (A) Viability of MDA-MB-231 cells after treatment with quercetin (50 and 200 μM) combined with doxorubicin (2, 8, and 32 nM) for 24h. (B) Viability of MDA-MB-231 cells after treatment with quercetin (50 and 200 μM) combined with doxorubicin (2, 8, and 32 nM) for 48 h. The results are presented as mean ± SEM of at least 3 independent experiments *P < 0.05; **P < 0.01; ***P < 0.001 significant from control untreated cells, and #P < 0.05; ## P < 0.01 significant from doxorubicin -alone treated cells.
| Dox (nM) | Que (µM) | CI |
|---|---|---|
| 2 | 50 | 0.4 |
| 8 | 50 | 0.38 |
| 32 | 50 | 0.36 |
| 2 | 200 | 0.88 |
| 8 | 200 | 0.82 |
| 32 | 200 | 0.65 |
4.2. Que Synergizes with Dox on Inhibition of Cancer Cell Migration
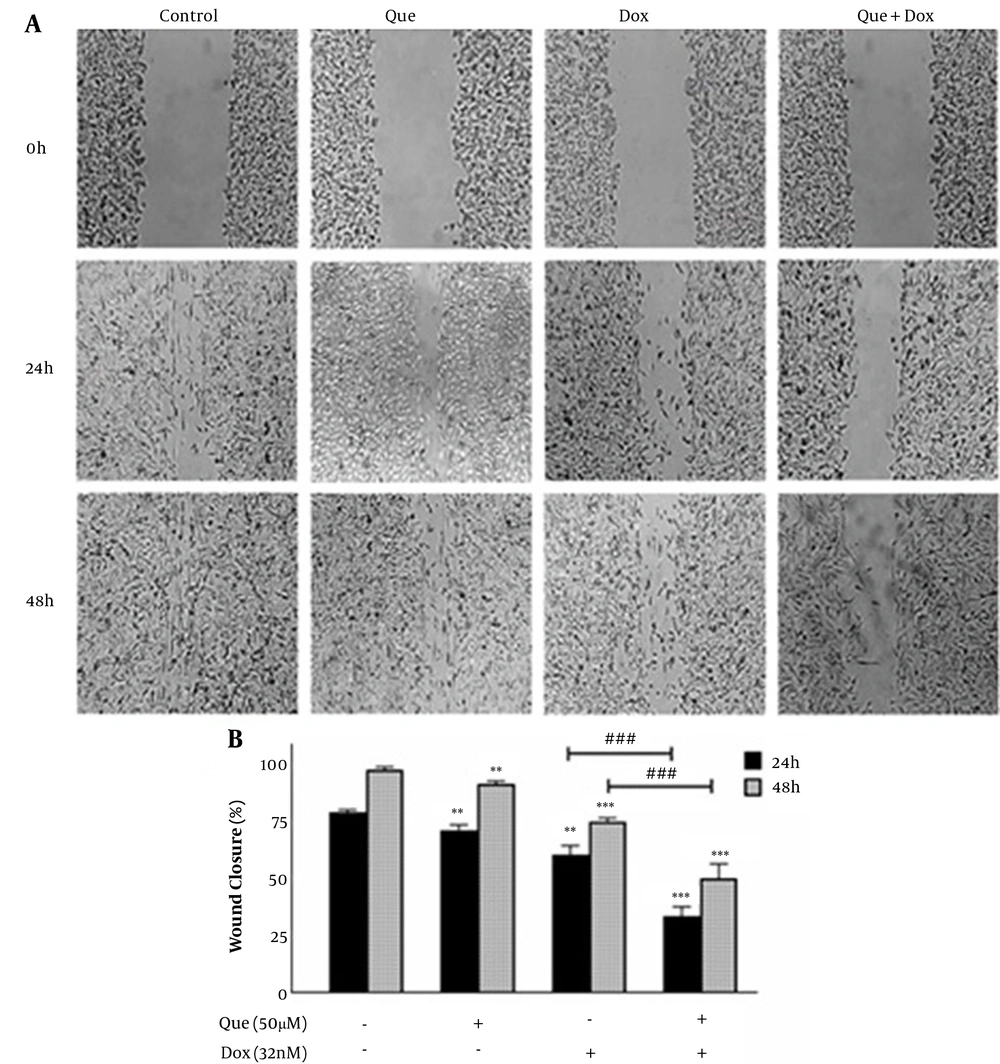
Cancer cells were treated with 50 µM of que and 32 nM of dox alone and in combination for 48 h. Figure 4 shows that both que and dox significantly reduced the migration of cancer cells to 92.5% and 76%, respectively. Besides, after treatment with the selected combination state, cancer cell migration was reduced to 50%, which shows a significant reduction compared to individual drugs.
Wound healing assay of MDA-MB-231 breast cancer cell line treated with doxorubicin and quercetin. (A) Image of MDA-MB-231 cells migration following treatment with quercetin (50 μM), doxorubicin (32 nM), and their combination for 48 h. (B) Quantitative analysis of the anti-migratory effect of quercetin (50 μM), doxorubicin (32 nM), and their combination for 48 h. The results are presented as mean ± SEM of at least 3 independent experiments **P < 0.01; ***P < 0.001 significant from control untreated cells, and ### P < 0.001 significant from doxorubicin -alone treated cells.
4.3. Que Enhanced the Effects of Dox on the Expression of MMP-2 and MMP-9 Genes
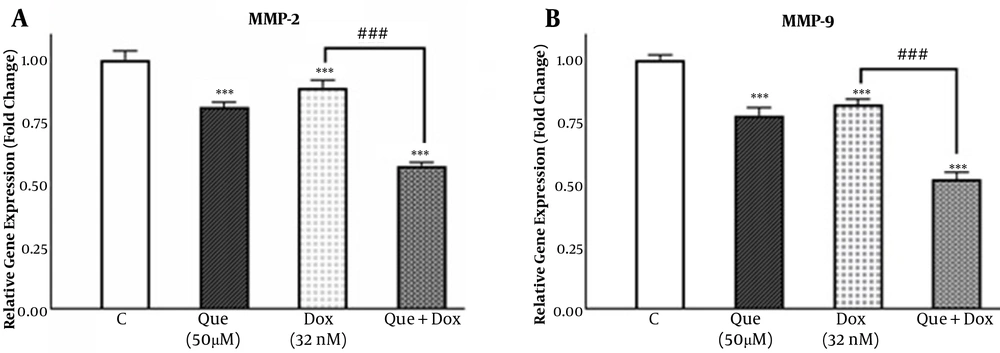
MDA-MB231 cells were treated with 50 µM of que and 32 nM of dox alone and in combination (50 µM que + 32 nM dox) for 48 h. Figure 5A shows that que and dox reduced the expression of the MMP-2 gene to 0.8 and 0.88 fold, respectively. Also, after treatment with the selected combination state, the expression of the MMP-2 gene was reduced to 0.57 fold. According to Figure 5B, the expression of the MMP-9 gene was decreased by que and dox to 0.77 and 0.82 fold, respectively, while when treated with the selected combination state, it was decreased to 0.52 fold, which suggests a synergistic effect between que and dox on the expression of MMP-2 and MMP-9 genes.
Quercetin enhanced the effect of doxorubicin on the matrix metalloproteinase-2 and matrix metalloproteinase-9 genes expression in the MDA-MB-231 breast cancer cell line. (A) The expression of the MMP-2 gene was evaluated in MDA-MB-231 untreated control, treated with quercetin (50 μM), doxorubicin (32 nM), and the combination of quercetin plus doxorubicin using real-time qPCR. (B) Expression of MMP-9 gene was evaluated in MDA-MB-231 untreated control, treated with quercetin (50 μM), doxorubicin (32 nM), and the combination of quercetin plus doxorubicin using quantitative real-time PCR. The results are presented as mean ± SEM of at least 3 independent experiments ***P < 0.001 significant from control untreated cells, and ###P < 0.001 significant from doxorubicin -alone treated cells.
5. Discussion
We investigated the effects of que as a natural substance in combination with the chemotherapeutic drug dox on the viability and migration of MDA-MB-231 breast cancer cells. Our results showed a synergistic suppressive effect between que and dox on the viability and migration of cancer cells.
The MTT assay results showed that que and dox individually decrease the viability of cancer cells. The cancer cell viability was significantly decreased when treated with the combination of que and dox compared to each drug alone, showing a synergistic effect between que and dox on cancer cell viability. The combination of 50 µM of que with 32 nM of dox with the lowest CI (0.36) and the highest synergy level was selected for the rest of the study, which had no significant effects on normal cells. According to our SI results, que had a more prominent impact on the viability of cancer cells rather than the normal cells. However, the same doses of dox have inhibited the normal cell rather than cancer cell viability. The difference could be due to the chemoresistance formed in cancer cells, which prevents dox from performing its inhibitory effects (24). On the other hand, the cytotoxic anticancer substances have a greater influence on more proliferative cells, which could be why que inhibits the proliferation of cancer cells more than normal cells (25). All DRI values were > 1, which indicates a reduced dose of dox in every combination state. According to our results, que enhances dox effects and simultaneously reduces its cytotoxicity on normal cells. It can be due to the antioxidant effects of que, which protects the cells against free radicals generated through dox activity (26). Also, the enhanced effects of dox in combination with que could be a result of the chemosensitizing ability of que, which impairs the cancer cell resistance to dox (18). Our results are in line with previous work on the synergistic effects of que in combination with chemotherapeutic agents and other natural compounds on the viability of cancer cells (27, 28).
The results of the wound healing assay showed that the migration of cancer cells treated with que and dox was reduced by 8% and 25% compared to the untreated control group. Also, the selected combination state reduced the cell migration to 50% compared to the control group and 42% and 25% compared to que and dox alone treated groups, respectively. These effects could be due to the dual role of que, which reduces the migration of cancer cells itself and increases the sensitivity of these cells to dox, thus enhancing the anti-migratory effects of dox (29, 30). These results are in agreement with previous studies about the effects of que alone and combined with other anticancer agents on cancer cell migration (31, 32).
The ability of que to alter the expression of different genes in normal and cancer cells has been studied. It is known that que has definitive impacts on important signaling pathways such as PI3K and ERK pathways, which finally lead to certain transcription factors (15, 16). The results of real-time PCR showed that que and dox reduced the expression of the MMP-2 gene to 0.8 and 0.88 fold, respectively, while the combination of que and dox decreased the MMP-2 gene expression to 0.57 fold, indicating a 23% and 31% reduction compared to the treatment with que and dox. After treatment with que and dox, the MMP-9 gene expression was reduced to 0.77 and 0.82 fold, respectively. However, the combination of que and dox reduced the expression of this gene to 0.51 fold, suggesting a reduction of 26% and 31% compared to the treatment with que and dox, respectively. Our results show that both que and dox can affect the migration of MDA-MB-231 cells by reducing metastasis-related genes expression and that que significantly enhances the inhibitory effects of dox on the expression of these genes. Our results agree with previous research on the inhibition of gelatinases gene expression by que and dox and the combination of these agents with different natural and chemotherapeutic agents (33, 34).
5.1. Conclusions
Our results suggest that que co-delivered with dox leads to a reduced dose of the chemotherapeutic drug, reducing its cytotoxicity on normal cells. Also, this combination can serve as a novel therapeutic agent in the inhibition of breast cancer metastasis. Nevertheless, this path needs further research to be more illuminated.