1. Background
Endometrial cancer (EC) is known as one of the most common malignancies of the female reproductive system (1). Most cases with this condition are also sporadic; however, germline mutations are present in up to 25% of patients, resulting in the occurrence of cancer in younger age groups (2). Mutation in one of the DNA mismatch repair (MMR) genes (including MLH1, MSH2, MSH6, or PMS2), i.e., hereditary nonpolyposis colorectal cancer (HNPCC) or Lynch syndrome (LS), is thus considered as the leading cause of inherited ECs (3). The inactivating mutations in the DNA MMR genes lead to a significant increase in the risk of endometrial cancer in the affected population (4-7).
DNA fragment analysis technique using capillary electrophoresis (CE) and denaturing high-performance liquid chromatography (DHPLC) is accordingly among the standard methods applied to confirm the diagnosis of LS through showing mutations in the DNA MMR genes (8-11) although, the assessment of the expression of MLH1, MSH2, MSH6, and PMS2 proteins using immunohistochemistry (IHC) is more widely available (12). IHC for a 4-antibody panel of the MMR proteins is also a highly sensitive and specific method to evaluate LS with a sensitivity between 85% and 100%, specificity between 85% and 92%, and overall, the concordance rate of 98% between MMR IHC and microsatellite instability (MSI) molecular testing (13, 14).
Despite recent progress in the molecular aspects of cancer biology, which sheds light on its underlying mechanisms and provides the opportunity to treat this condition more effectively (15-19), the data regarding the presence of mutations in the DNA MMR genes in the Iranian female population with EC is extremely limited (20).
2. Objectives
The aim of the present study was to screen for LS using IHC in patients with EC.
3. Methods
3.1. Clinical Samples
In this cross-sectional study, the formalin-fixed paraffin-embedded tissue specimens of women with EC which were submitted to the Department of Pathology at Qaem Hospital affiliated to Mashhad University of Medical Sciences, Mashhad, Iran, in 2015 - 2019, were examined. The inclusion criterion was the primary diagnosis of EC regardless of its histological subtypes. The given specimens were also excluded if there was not enough tumoral tissue, inappropriate fixation and proccing based on internal negative control, being metastatic at the presentation [considering the rarity of condition for patients with endometrial cancer], and not having access to patients for follow-up purposes. Moreover, this work was approved by the Institutional Review Board of Mashhad University of Medical Sciences, Mashhad, Iran, and all the specimens were collected after obtaining written informed consent from the patients.
3.2. Pathological Assessment and IHC
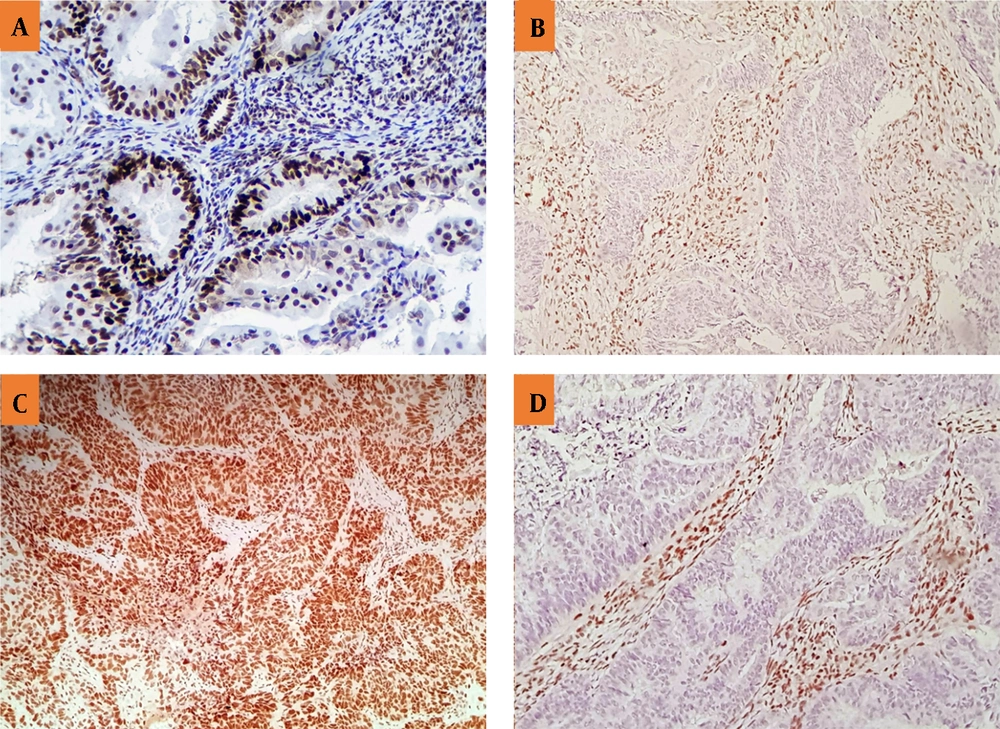
Mouse anti-human MLH1, MSH2, MSH6, and PMS2 monoclonal antibodies (Master Diagnóstica Co., Spain) were utilized to assess the expression of the corresponding MMR proteins. The staining procedures had been also previously described in detail for this purpose (21). Moreover, two independent pathologists evaluated the stained specimens and a third opinion was requested if there was any discordance. In the absence of the nuclear staining of any of the MMR proteins, LS was suspected. The nuclear immunoreactions of lymphocytes and stromal cells additionally served as positive controls (Figure 1).
The IHC of MSH2 (A), PMS2 (B), MSH6 (C), and MLH1 (D). A and C: Positive nuclear staining for MSH2 and MSH6, showing the expression of the MMR proteins or the positive results. B and D: Negative nuclear staining for PMS2 and MLH1 along with positive internal control (positive staining for inflammatory and stromal cells), showing no expression of the MMR proteins or negative results.
3.3. Statistical Analysis
The sample size was determined by 100 cases according to Rabban et al. (22), using the prevalence formula with a relative accuracy of 25% and a prevalence of 44% for the MMR loss. The data were also analyzed using the IBM SPSS Statistics software (ver.21) and the chi-square test, the independent-samples t-test, and the Mann-Whitney U test at the significance level of P < 0.05.
4. Results
The total number of patients undergoing hysterectomy because of the malignant lesion of the uterine endometrium, referred to Qaem Hospital, Mashhad, Iran, between 2015 and 2019, was 168, of which 27 cases were diagnosed with metastases, choriocarcinoma, and endometrial stromal tumors (ESTs). Out of 141 remaining patients with primary ECs, 34 cases were also excluded from the study due to the lack of enough tissue block or inappropriate tumor volume in the tissue block. IHC was further performed on 107 samples, of which seven cases were excluded because of negative internal control staining following two attempts. Finally, in this study, 100 patients with EC were evaluated for IHC tumor markers. Table 1 shows the demographic characteristics of the patients with EC.
| Variables | Frequency |
|---|---|
| Age | |
| < 50 years old | 70 |
| > 50 years old | 30 |
| Previous history of malignancies | 7 |
| Breast cancer | 4 |
| Ovarian cancer | 1 |
| Breast and ovarian cancers | 1 |
| Squamous cell carcinoma of cervix | 1 |
| Family history of malignancies a | 14 |
| Gastrointestinal cancers | 6 |
| EC | 4 |
| Breast cancer | 3 |
| Ovarian cancer | 1 |
| Tumor site | |
| Lower uterine segment | 22 |
| Other places | 78 |
| Tumor type | |
| Endometroid carcinoma | 90 |
| MMMT | 8 |
| Clear cell carcinoma | 2 |
| Tumor grade | |
| I | 50 |
| II | 25 |
| III | 25 |
Abbreviations: EC, endometrial cancer; MMMT, malignant mixed Mullerian tumor.
a Five patients had one or more family history of malignancies.
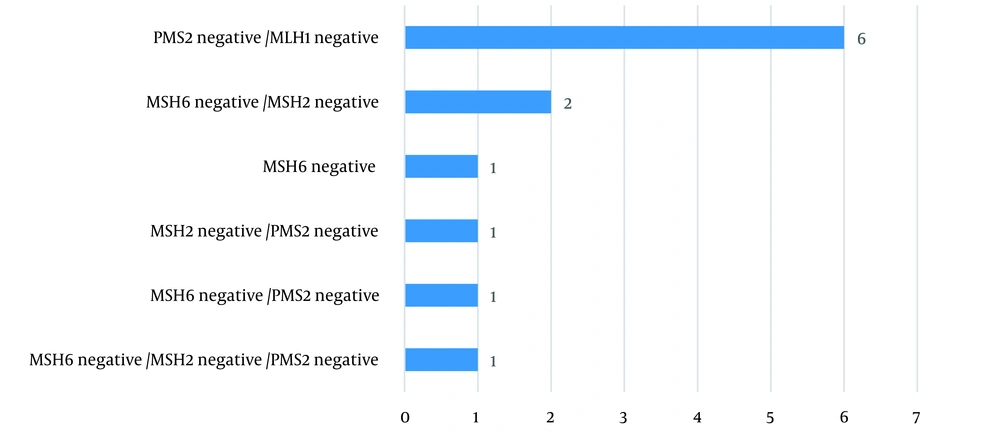
The lack of expression of at least one of the MMR proteins was reported in 12 patients (Table 2). Moreover, the most predominant pattern was the loss of MLH1/PMS2 expression (Figure 2).
| Variables | Frequency |
|---|---|
| Loss of MSH6 expression | 5 |
| Loss of PMS2 expression | 9 |
| Loss of MSH2 expression | 4 |
| Loss of MLH1 expression | 6 |
There was also no significant relationship between the aberrant expression of the MMR proteins and age (P = 0.283), tumor site (P = 0537), tumor histology (P = 0.469), tumor grade (P = 0.408), and a family (P = 0.242) and personal (P = 0.162) history of malignancies (Table 3).
| Variables | The Lack of Expression of at Least One of the MMR Proteins | The Expression of All of the MMR Proteins | P-Value |
|---|---|---|---|
| Age | 0.283 | ||
| < 50 years old | 2 (16.7) | 28 (31.8) | |
| > 50 years old | 10 (83.3) | 60 (68.2) | |
| Tumor site a | 0.537 | ||
| Lower uterine segment | 2 (20) | 20 (29.4) | |
| Other places | 8 (80) | 48 (70.6) | |
| Tumor type | 0.469 | ||
| Endometroid carcinoma | 12 (100) | 78 (88.6) | |
| MMMT | 0 | 8 (9.1) | |
| Clear cell carcinoma | 0 | 2 (2.3) | |
| Tumor grade | 0.408 | ||
| High | 2 (16.7) | 78 (34.1) | |
| Moderate | 10 (83.3) | 8 (64.8) | |
| Low | 0 | 2 (1.1) | |
| Family history of malignancies | 3 (25) | 11 (12.5 | 0.242 |
| Personal history of malignancies | 2 (16.7) | 5 (5.7) | 0.162 |
a Data of two patients in column “the lack of ... MMR proteins” and data 20 patients in in column “the expression of ... proteins” are missing.
5. Discussion
HNPCC or LS is mainly associated with the types of cancer affecting the gastrointestinal and female genital tracts. In this sense, EC is known as the most common malignancy of the female reproductive system, which has been suggested to be associated with LS. The gold standard in the assessment of LS is to detect molecular alterations in genes encoding the MMR proteins, which is often a costly method and is not widely available, especially in developing countries. Therefore, this diagnostic method is not suitable for screening purposes. On the other hand, previous studies have thus far shown a good correlation between IHC results (as an available and relatively cheaper method) and polymerase chain reaction (PCR) test ones in the evaluation of LS with an overall concordance rate of 98% between MMR IHC and MSI molecular testing (13, 14, 23). The easiest diagnostic tool in the study of MMR expression impairment is also tissue staining for proteins (i.e., against MLH1, MSH2, MSH6, and PMS2), which can be performed in most pathology laboratories based on some standard protocols. The use of IHC also allows physicians to examine either the expression or lack of expression of the MMR proteins; however, it fails to provide further information on their activity. Gene mutations also lead to the production of abnormal proteins, and IHC can detect the absence of one or more of them (24, 25). Although a definitive diagnosis of LS requires a next-generation sequencing (NGS) of the genes to detect germline mutations in one of the MMR proteins, information from IHC can be helpful in evaluating the targeted genes.
The present study was to screen for LS using IHC in patients with EC. To this end, the reported frequency of LS based on the IHC of the MMR protein expression was 12%. As well, no significant relationship was observed between the cases suspected of this syndrome and the demographic and tumor-related data. The examination of the MMR proteins in patients with EC had been also considered by various researchers in Iran and other parts of the world. In a study conducted by Abbaszadegan et al., 23 patients with EC in the age group younger than 55 years in Mashhad, Iran, had been accordingly assessed for MSI by the PCR test and the results demonstrated high and low levels of MSI (viz. MSI-H and MSI-L) phenotypes in 47.8% and 43.4% of the cases, respectively. The mean age of the patients with MSI-H was also higher than that of the ones with MSI-L (i.e., 48 vs. 45.5 years old). As well, there was no relationship between the MSI status and contraceptive pill use, pregnancy, underlying diseases, and menopausal status (20). Despite enrolling the same population, the frequency of the patients with LS in Abbaszadegan et al. (20) was significantly higher than the ones recruited in the present study. Moreover, they had purposefully examined the patients with EC, under 55 years of age, so that the mean age of the patients was 48. In addition to the smaller sample size in the given study, they had additionally recruited a different diagnostic tool (namely, a PCR test), which both might contribute to the discrepancy of the results. To the best of the authors’ knowledge, there was no other study in this context in Iran.
In Egoavil et al., the abnormal expression of the MMR proteins had been similarly observed in 35% (out of 173 patients) of new patients with EC. However, after the study of MLH1 methylation, there were 27 patients suspected of LS, which was finally confirmed in only eight patients after the genetic evaluation of this condition (26). In a pathological study of 98 patients with sporadic EC in 2010 - 2019 in Tokyo, Japan, using IHC for the MMR proteins (including MLH1, MSH2, MSH6, and PMS2), the patients had been simultaneously assessed by the PCR test for MSI. The lack of expression of at least one MMR protein had been also reported in 23.5% of the patients. As well, the highest non-expression related to MLH1/PMS2, MHS6/MSH2, and MSH6 had been 14.3%, 4.1%, and 4.1%, respectively. Moreover, the frequency of MSI-H had been 10.2%, and this value had been 8.2% and 81.6% for MSI-L and microsatellite stable (MSS), respectively. In patients with MSI-H, the frequency of the tumors with the loss of MMR proteins (P = 0.001) and high malignancy had been significantly higher. Furthermore, no relationship had been observed between the MSI status and the estrogen (ER) status and the International Federation of Gynecology and Obstetrics (FIGO) stage. However, there was a significant relationship between MSI-H and the loss of MMR proteins (27). In the present study, the lack of expression of at least one protein was reported in 12% of the patients, which had a similar pattern to that illustrated in Saeki et al (27).
In Sarode and Robinson, LS screening had been done retrospectively using IHC for the expression of the MSI proteins in the specimens of patients with colorectal and endometrial cancers (28). The expression and lack of expression of MSS had been thus reported in 78 and 21 patients with EC, respectively. In another study, Chapel et al. examined the correlation between the IHC results of MSS in biopsy and hysterectomy specimens, using the data from the patients who underwent a hysterectomy, and revealed that the IHC results associated with the biopsy were completely consistent with the hysterectomy ones (29). Of the 99 patients examined, the absence of MLH1 and PMS2 had been also observed in 26 cases, there were no MSH2 and MSH6 in three patients, and no isolated PMS2 had been detected in one patient. In addition, the MMR protein-retained had been reported in 69 patients. The FIGO stage in the cases with MMR protein-deficient tumors had been also significantly higher than that in the MMR protein-retained ones (P = 0.004). Other demographic and tumor-related data were not also connected with the MSS status.
In addition to the role of MSI in determining the likelihood of LS, the study on patients with EC today has other roles such as patient classification and predictions of the effectiveness of cancer treatments (29). Besides MSI, recent evidence has delineated the functional role of mitochondria in repairing DNA mutations as well as its crucial role in cancer pathogenesis and their responses to treatments (30). Moreover, alteration of the K-Ras gene and other tumor suppressor genes are among the other important pathways (31, 32).
The strength of the present study was also some reflections on the expression of MLH1, MSH2, MSH6, and PMS2 proteins in a suitable sample size of patients. The lack of a PCR test to investigate gene mutations with the genes encoding MLH1, MSH2, MSH6, and PMS2 proteins was thus a limitation facing this study. Another limitation was the absence of prospective follow-up of the patients in terms of the occurrence of subsequent malignancies or the evaluation of the therapeutic effectiveness of adjuvant prescriptions. Similar research in other malignancies, such as those with breast and ovarian cancers, is accordingly necessary. It is also suggested to evaluate the genetic variation of the genes responsible for the MMR proteins by the PCR test in patients with these cancers in future studies. Considering the predictive role of the MMR proteins in determining the effectiveness of treatments and their role in the prognosis of patients, prospective follow-up of the cases suspected with LS in terms of subsequent malignancies, the disease outcomes, and the effectiveness of the treatments applied are essential. Screening family members of these patients is also one of the suggestions for future studies.
5.1. Conclusions
HNPCC or LS is mainly associated with cancers affecting the gastrointestinal and female genital tracts. In this regard, EC is the most common malignancy of the female reproductive system, which has been suggested to be associated with LS. Therefore, the present study was to screen for LS using IHC in patients with EC. In this study, 100 patients with EC were thus evaluated for IHC tumor markers. In 12 (12%) patients, LS was also suspected based on the IHC results for the MMR protein expression. There was also no significant relationship between the cases suspected with LS and age, tumor site, tumor histology, tumor size, tumor grade, tumor-infiltrating lymphocytes (TILs), and a family/personal history of malignancies.