1. Background
Chondrosarcoma is a primary malignant bone neoplasm (1) caused due to multi-potential mesenchymal precursor cells (2). These neoplasms are characterized by the formation of a cartilage matrix (3). Chondrosarcoma is regarded as the second most common primary bone malignancy following osteosarcoma (4). The prevalence of chondrosarcoma increases with age (5). It has been reported that the incidence of chondrosarcoma has increased from 2.88 to 8.78 per million people in recent years (6). Chondrosarcoma mostly affects long bones of the upper and lower limb and pelvic bone (7) and is classified into 6 subtypes, including conventional, juxtacortical, myxoid, mesenchymal, clear cell, and dedifferentiated (4, 8-10). The conventional subtype is the most common, while the other subtypes are relatively rare (4). Prognosis of these subtypes significantly varies such that the dedifferentiated subtype has the worst prognosis with a 5-year survival rate of 18% (11) and the clear cell subtype has the best prognosis with a 5-year survival rate of 100% with adequate treatment (12). Chondrosarcomas also have 3 histological grades: (1) low (grade 1), (2) high (grade 2), and (3) THE presence of metastasis (grade 3) (13-15). Surgical resection is the main treatment for Chondrosarcoma, especially for low-grade tumors (5). Surgical treatment is most effective for low-grade chondrosarcoma as recurrence and metastasis are common after the surgical resection of high-grade tumors (16).
There are several studies on the survival of patients with chondrosarcoma. Patients’ 5-year survival rate varies from 64.4% to 78% in different studies (7, 17, 18). Several factors affect the prognosis of patients with chondrosarcoma. Male gender, older ages, higher stage and grade of the disease, larger tumor size, and poor socioeconomic status are related to worse prognosis (18, 19). Tumor grade and surgical stage of the tumor have the highest association with the disease’s prognosis (20).
Several studies have been conducted on epidemiology, surveillance, and chondrosarcoma outcomes in different countries, but there is no data available on chondrosarcoma in Iran and middle-east countries.
2. Objectives
3. Methods
3.1. Study Population
This investigation was a population-based study conducted, using the INCR data. The Ethics Committee of Shahid Beheshti University of Medical Sciences approved the study protocol (ethics code: IR.SBMU.CRC.1398.010). Nearly all patients diagnosed with any cancer have been registered in INCR from all over the country. All patients with chondrosarcoma, who had been registered in the INCR from March 20, 2008, to March 20, 2015, were included in the study. The diagnosis of chondrosarcoma was confirmed based on pathological findings in these patients. The current data was the last available and ready data to be analyzed since data collection, recording, preparation, cleaning, and review of data were very time-consuming.
The data available included problems after the collection such as incorrect age of patients, day of diagnosis, and recording wrong histology of the disease. In addition, the completeness of data was not checked. However, after completing data collection, the initial evaluation of data qualification was performed by INCR in several steps as follows: the consistency of histology and morphology of each case was assessed. Accordingly, if irrelevant topography or histology was present in the data, that case would be corrected. Furthermore, the diagnosis of chondrosarcoma was evaluated, and if the diagnosis was incorrect or invalid, the data would be re-examined or excluded for further evaluation. In addition, any inconsistencies between the patient’s birthday and date of cancer diagnosis were evaluated and re-examined. The completeness of data was evaluated by INCR, using the capture-recapture method (23).
We excluded patients that we could not contact and those whose quality criteria did not meet in the study. Those with identical first names, surnames, and fathers’ names were considered duplicated cases, and one of them was excluded from the study.
3.2. Data Variables
We recorded the patients’ demographic characteristics such as age, gender, and city of living. We used the third version of the International Classification of Diseases for Oncology (ICD-O-3) to classify the patients based on the chondrosarcoma subtype and the area that had been affected (24). Chondrosarcomas were divided into 6 histological subtypes according to ICD-O-3 and a unique M-code was dedicated to each subtype including 9220 (conventional chondrosarcoma), 9221 (juxtacortical chondrosarcoma), 9231 (myxoid chondrosarcoma), 9240 (mesenchymal chondrosarcoma), 9242 (clear cell chondrosarcoma), and 9243 (dedifferentiated chondrosarcoma). Another code was dedicated to tumors (C-code) based on the tumors' primary site; C40 (tumors of the limb) and C41 (tumors in other areas and unspecified sites). The incidence rate of chondrosarcoma cancer was calculated, using national census data provided by statistical cancer of Iran in 2006, 2011, and 2016. The population of other years was obtained through a growth rate between 2 consecutive censuses. Accordingly, each year’s population was calculated by multiplying the population of the previous year by the growth rate.
Telephone interviews as a valid and suitable method (25) were conducted with all patients to check and verify the type of cancer. In addition, the survival status, death status due to chondrosarcoma cancer, and patients’ death date were questioned and recorded.
3.3. Statistical Analysis
For descriptive analysis, frequency and percentage were calculated for categorical variables, and mean, and standard deviation (SD) were calculated for continuous variables. The proportion of women with chondrosarcoma was compared with that of men by the binomial proportion test.
The crude incidence rate (per million) and 95% confidence intervals (CIs) were obtained as follows:
Where r is the number of new cases in ith age group and n is the person-years of observation. Furthermore, the age-standardized incidence rate (ASIRs) per million was calculated, using the direct standardization method and the World Health Organization’s (WHO) new standard population (WHO 2000 - 2025) per million person-years as follows (26, 27):
Where is the crude incidence rate in each age category and is the new WHO standard population.
In this study, mortality was defined as patients’ death due to chondrosarcoma interval between chondrosarcoma diagnosis, and death in these patients was considered survival time. The Kaplan-Meier survival method was used to estimate the 1, 3, 5, and 7-year survival rates for each sex, the decade of age, and topographies, respectively. Also, the survival curve was plotted for overall patients and each topography code. P-value ≤ 0.05 was set as the significance level. We used SPSS software (version 25) for data analysis.
4. Results
A total of 750 patients were included in the study, 2.40% of whom were identified as duplicated and were removed (Table 1). All cancer diagnosis was microscopically confirmed (pathology diagnosis) in chondrosarcoma. The demographic characteristics of participants and ASIR for all morphological subtypes are summarized in Table 2. Out of 732 enrolled patients, 425 cases (58.06%) were male and 307 (41.94%) were female with a mean age of 44.08 years (SD = 19.31) and 45.06 years (SD = 18.72), respectively. The proportion of men with chondrosarcoma was higher than women (P < 0.01). Conventional chondrosarcoma was the most common subtype and constituted 84.7% of patients with chondrosarcoma. Myxoid, mesenchymal, dedifferentiated, clear cell, and juxtacortical chondrosarcoma constituted 5.6%, 4.78%, 2.86%, 1.36%, and 0.68% of patients with chondrosarcoma, respectively. The mean age of males and females was not significantly different in all morphological subtypes (P > 0.05). ASIR of chondrosarcoma was 1.5 (95% CI = 1.39 - 1.61) in 1 million. Conventional chondrosarcoma had the highest ASIR among morphological subtypes with an ASIR of 1.28 (95% CI = 1.18 - 1.38) in 1 million.
| Year | Before Duplicate Extraction | After Duplicated Extraction | Percentage |
|---|---|---|---|
| 2008 | 110 | 110 | 0.00 |
| 2009 | 116 | 116 | 0.00 |
| 2010 | 125 | 125 | 0.00 |
| 2011 | 101 | 95 | 5.94 |
| 2012 | 88 | 80 | 9.09 |
| 2013 | 116 | 112 | 3.45 |
| 2014 | 94 | 94 | 0.00 |
| Total | 750 | 732 | 2.40 |
Percentage of Data Extraction
| M-Code | Morphology Type | Number of New Cases (Percentage) | Mean Age (Standard Deviation) | ASIR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | Total | Male | Female | ||
| 9220 | Conventional Chondrosarcoma | 620 (100.0) | 365 (58.87) | 255 (41.13) | 45.32 (18.78) | 44.95 (19.49) | 45.86 (17.75) | 1.28 (1.18 - 1.38) | 1.50 (1.34 - 1.66) | 1.06 (0.93 - 1.19) |
| 9221 | Juxtacortical chondrosarcoma | 5 (100.0) | 5 (100.0) | 0 (0.00) | 32.40 (12.34) | 32.40 (12.34) | 0 (0.00) | 0.01 (0.00 - 0.02) | 0.02 (0.00 - 0.03) | 0.00 (0.00 - 0.00) |
| 9231 | Myxoid chondrosarcoma | 41 (100.0) | 23 (56.10) | 18 (43.90) | 42.41 (22.02) | 41.83 (19.49) | 43.17 (25.47) | 0.08 (0.06 - 0.11) | 0.09 (0.05 - 0.13) | 0.07 (0.04 - 0.11) |
| 9240 | Mesenchymal chondrosarcoma | 35 (100.0) | 14 (40.00) | 21 (60.00) | 32.06 (16.82) | 33.36 (17.01) | 31.19 (17.05) | 0.06 (0.04 - 0.09) | 0.05 (0.02 - 0.08) | 0.08 (0.04 - 0.11) |
| 9242 | Clear cell chondrosarcoma | 10 (100.0) | 3 (30.00) | 7 (70.00) | 52.10 (20.54) | 44.33 (17.50) | 55.43 (22.08) | 0.02 (0.01 - 0.04) | 0.01 (0.00 - 0.03) | 0.03 (0.01 - 0.06) |
| 9243 | Dedifferentiated chondrosarcoma | 21 (100.0) | 15 (71.43) | 6 (28.57) | 44.05 (17.89) | 40.33 (16.27) | 53.33 (19.85) | 0.04 (0.02 - 0.06) | 0.06 (0.03 - 0.09) | 0.03 (0.00 - 0.05) |
| Total a | 732 (100.0) | 425(58.06) | 307 (41.94) | 44.49 (19.06) | 44.08 (19.31) | 45.06 (18.72) | 1.50 (1.39-1.61) | 1.73 (1.56 - 1.90) | 1.27 (1.12 - 1.41) | |
The Number of New Cases, ASIR, and Mean Age (Standard Deviation) of Patients with Chondrosarcoma from 2008 to 2014 in Iran
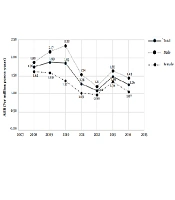
The age-specific incidence rate of chondrosarcoma and its 3 more prevalent subtypes, conventional, myxoid, and mesenchymal, per million person-years among the Iranian population are shown in Table 3. Results showed that 71.03% of all Chondrosarcoma patients (70.35% of males and 71.99% of females) were between 20 and 59 years old. Similar patterns were extracted for conventional, myxoid, and mesenchymal chondrosarcoma such that 70.97%, 73.17%, and 68.57% of patients in these subtypes were 20 to 59 years. Chondrosarcoma and its conventional subtype were more common among males, who were 20 to 24 years than females of the same age (P < 0.05). ASIR of chondrosarcoma by gender from 2008 to 2014 is shown in Figure 1.
| Age (Decade) | Conventional Chondrosarcoma | Myxoid Chondrosarcoma | Mesenchymal Chondrosarcoma | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | Total | Male | Female | Total | Male | Female | |
| 0 - 4 | 8 (0.18) | 5 (0.22) | 3 (0.14) | 3 (0.07) | 2 (0.09) | 1 (0.05) | 1 (0.02) | 0 (0.00) | 1 (0.05) | 12 (0.27) | 7 (0.31) | 5 (0.23) |
| 5 - 9 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| First | 8 (0.09) | 5 (0.12) | 3 (0.07) | 3 (0.04) | 2 (0.05) | 1 (0.02) | 1 (0.01) | 0 (0.00) | 1 (0.02) | 12 (0.14) | 7 (0.16) | 5 (0.12) |
| 10 - 14 | 6 (0.15) | 5 (0.24) | 1 (0.05) | 1 (0.02) | 0 (0.00) | 1 (0.05) | 2 (0.05) | 1 (0.05) | 1 (0.05) | 9 (0.22) | 6 (0.29) | 3 (0.15) |
| 15 - 19 | 24 (0.51) | 17 (0.71) | 7 (0.30) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 5 (0.11) | 2 (0.08) | 3 (0.13) | 30 (0.64) | 20 (0.84) | 10 (0.43) |
| Second | 30 (0.34) a | 22 (0.49) | 8 (0.18) | 1 (0.01) | 0 (0.00) | 1 (0.02) | 7 (0.08) | 3 (0.07) | 4 (0.09) | 39 (0.44) | 26 (0.58) | 13 (0.30) |
| 20 - 24 | 53 (0.93) a | 38 (1.33) | 15 (0.52) | 3 (0.05) | 1 (0.04) | 2 (0.07) | 6 (0.11) | 3 (0.11) | 3 (0.10) | 66 (1.16) a | 46 (1.61) | 20 (0.70) |
| 25 - 29 | 60 (1.03) | 36 (1.23) | 24 (0.83) | 5 (0.09) | 3 (0.10) | 2 (0.07) | 5 (0.09) | 1 (0.03) | 4 (0.14) | 74 (1.27) | 43 (1.46) | 31 (1.07) |
| Third | 113 (0.98) a | 74 (1.28) | 39 (0.68) | 8 (0.07) | 4 (0.07) | 4 (0.07) | 11 (0.10) | 4 (0.07) | 7 (0.12) | 140 (1.21) a | 89 (1.54) | 51 (0.88) |
| 30 - 34 | 48 (0.98) | 22 (0.89) | 26 (1.07) | 3 (0.06) | 1 (0.04) | 2 (0.08) | 2 (0.04) | 2 (0.08) | 0 (0.00) | 58 (1.19) | 28 (1.13) | 30 (1.24) |
| 35 - 39 | 51 (1.28) | 28 (1.38) | 23 (1.17) | 4 (0.10) | 3 (0.15) | 1 (0.05) | 4 (0.10) | 0 (0.00) | 4 (0.20) | 62 (1.56) | 33 (1.62) | 29 (1.48) |
| Forth | 99 (1.11) | 50 (1.11) | 49 (1.12) | 7 (0.08) | 4 (0.09) | 3 (0.07) | 6 (0.07) | 2 (0.04) | 4 (0.09) | 120 (1.35) | 61 (1.35) | 59 (1.35) |
| 40 - 44 | 62 (1.82) | 37 (2.14) | 25 (1.48) | 5 (0.15) | 3 (0.17) | 2 (0.12) | 3 (0.09) | 1 (0.06) | 2 (0.12) | 75 (2.20) | 44 (2.55) | 31 (1.85) |
| 45 - 49 | 49 (1.71) | 25 (1.74) | 24 (1.69) | 2 (0.07) | 2 (0.14) | 0 (0.00) | 2 (0.07) | 2 (0.14) | 0 (0.00) | 54 (1.89) | 30 (2.09) | 24 (1.70) |
| Fifth | 111 (1.77) | 62 (1.96) | 49 (1.58) | 7 (0.11) | 5 (0.16) | 2 (0.06) | 5 (0.08) | 3 (0.09) | 2 (0.06) | 129 (2.06) | 74 (2.34) | 55 (1.77) |
| 50 - 54 | 61 (2.52) | 36 (2.97) | 25 (2.07) | 2 (0.08) | 1 (0.08) | 1 (0.08) | 1 (0.04) | 1 (0.08) | 0 (0.00) | 64 (2.64) | 38 (3.14) | 26 (2.16) |
| 55 - 59 | 56 (3.03) | 30 (3.28) | 26 (2.79) | 6 (0.32) | 4 (0.44) | 2 (0.21) | 1 (0.05) | 0 (0.00) | 1 (0.11) | 67 (3.63) | 37 (4.05) | 30 (3.21) |
| Sixth | 117 (2.74) | 66 (3.10) | 51 (2.38) | 8 (0.19) | 5 (0.24) | 3 (0.14) | 2 (0.05) | 1 (0.05) | 1 (0.05) | 131 (3.07) | 75 (3.53) | 56 (2.62) |
| 60 - 64 | 35 (2.64) | 17 (2.67) | 18 (2.62) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.08) | 0 (0.00) | 1 (0.15) | 38 (2.87) | 19 (2.98) | 19 (2.75) |
| 65 - 69 | 29 (3.04) | 21 (4.50) | 8 (1.64) | 1 (0.10) | 1 (0.21) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 31 (3.22) | 22 (4.72) | 9 (1.81) |
| Seventh | 64 (2.81) | 38 (3.44) | 26 (2.22) | 1 (0.04) | 1 (0.09) | 0 (0.00) | 1 (0.04) | 0 (0.00) | 1 (0.09) | 69 (3.03) | 41 (3.71) | 28 (2.39) |
| 70 - 74 | 30 (3.81) | 18 (4.52) | 12 (3.09) | 4 (0.51) | 2 (0.50) | 2 (0.52) | 2 (0.25) | 1 (0.25) | 1 (0.26) | 40 (5.06) | 22 (5.52) | 18 (4.59) |
| 75 - 79 | 23 (3.77) | 14 (4.41) | 9 (3.08) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 24 (3.95) | 14 (4.41) | 10 (3.45) |
| 80 - 85 | 18 (4.58) | 11 (5.51) | 7 (3.63) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 18 (4.59) | 11 (5.51) | 7 (3.63) |
| +85 | 7 (2.73) | 5 (3.80) | 2 (1.61) | 2 (0.78) | 0 (0.00) | 2 (1.61) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 10 (3.91) | 5 (3.79) | 5 (4.03) |
| Total | 620 (1.18) a | 365 (1.38) | 255 (0.98) | 41 (0.08) | 23 (0.09) | 18 (0.07) | 35 (0.07) | 14 (0.05) | 21 (0.08) | 732 (1.40) a | 425 (1.60) | 307 (1.84) |
The Number of New Cases (Age-Specific Incidence Rate/Per Million Person-Years) for Chondrosarcoma, Myxoid Chondrosarcoma, Mesenchymal Chondrosarcoma, and Total Records from 2008 to 2014
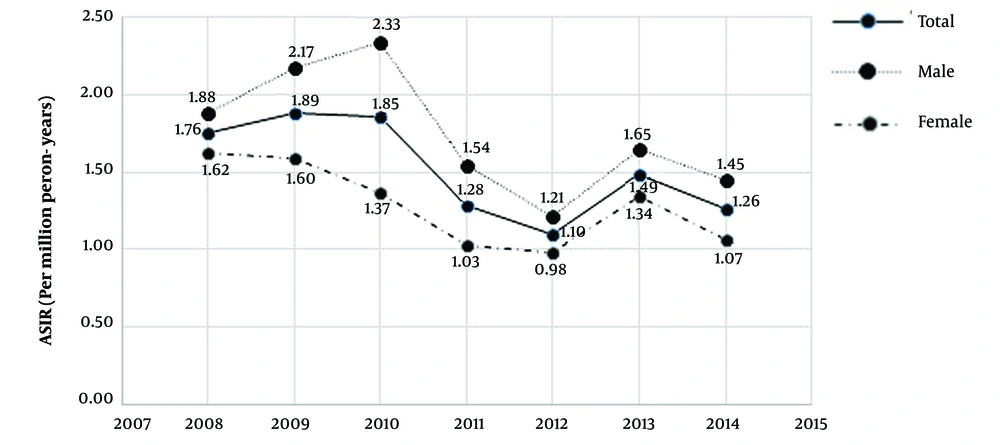
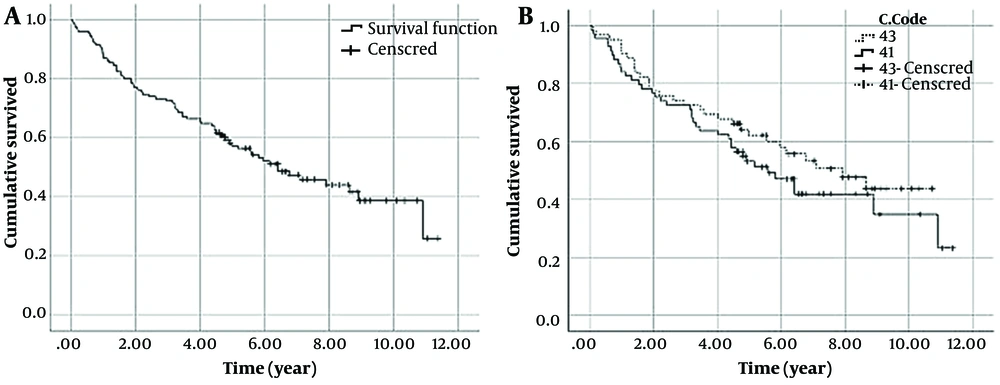
The outcome of 117 patients was used for evaluating the survival of patients with chondrosarcoma. The Kaplan-Meier curve showed that patients’ survival probability was about 0.25 at the end of the study. Besides, considering topographies, patients with a c-code of 40 had better survival than those with a c-code of 41 (Figure 2).
Mean and median survival time, 1-, 3-, 5-, and 7-year survival rates of patients with chondrosarcoma based on age, gender, m-code, and c-code are shown in Table 4. Also, 1-, 3, and 5-year survival rates of patients were 0.87 (95% C.I: 0.82 - 0.93), 0.73 (0.65 - 0.81), and 0.57 (0.39 - 0.58), respectively. Also, the mean survival time was 6.12 years (95% CI: 5.85 - 7.39) and the median survival time was 6.41 years.
| Variables | Survival Rate | Mean Survival Time (y) (95% C.I) | Median Survival Time (y) (95% C.I) | |||
|---|---|---|---|---|---|---|
| 1-Year Survival Rate (95% C.I) | 3-Year Survival Rate (95% C.I) | 5-Year Survival Rate (95% C.I) | 7-Year Survival Rate (95% C.I) | |||
| C-Code | ||||||
| 40 (n = 62) | 0.90 (0.83 - 0.98) | 0.73 (0.62 - 0.85) | 0.62 (0.51 - 0.76) | 0.53 (0.42 - 0.68) | 6.83 (5.81 - 7.86) | 7.19 (5.01 - 10.81) |
| 41 (n = 69) | 0.84 (0.76 - 0.93) | 0.73 (0.63 - 0.84) | 0.53 (0.43 - 0.67) | 0.42 (0.31 - 0.57) | 6.26 (5.20 - 7.33) | 5.59 (3.75 - 7.3) |
| Sex | ||||||
| Male (n = 85) | 0.84 (0.76 - 0.92) | 0.71 (0.62 - 0.81) | 0.56 (0.46 - 0.68) | 0.45 (0.35 - 0.59) | 6.40 (5.47 - 7.32) | 5.96 (4.01 - 7.91) |
| Female (n = 46) | 0.94 (0.87 - 1.00) | 0.76 (0.65 - 0.90) | 0.60 (0.65 - 0.89) | 0.50 (0.37 - 0.69) | 8.86 (5.57 - 8.15) | 7.91 (4.07 - 11.76) |
| M-Code | ||||||
| Conventional chondrosarcoma (n = 107) | 0.88 (0.82 - 0.94) | 0.71 (0.63 - 0.80) | 0.57 (0.48 - 0.70) | 0.45 (0.36 - 0.56) | 6.48 (5.64 - 7.32) | 6.15 (4.65 - 7.66) |
| Decade of age | ||||||
| Third (n = 32) | 0.91 (0.81 - 1.00) | 0.72 (0.58 - 0.89) | 0.62 (0.47 - 0.82) | 0.51 (0.35 - 0.75) | 7.16 (5.65 - 8.66) | - |
| Forth (n = 20) | 0.85 (0.71 - 1.00) | 0.80 (0.64 - 1.00) | 0.75 (0.58 - 0.97) | 0.63 (0.44 - 0.89) | 6.58 (5.06 - 8.10) | - |
| Fifth (n = 24) | 0.92 (0.81 - 1.00) | 0.79 (0.65 - 0.97) | 0.67 (0.50 - 0.89) | 0.60 (0.42 - 0.85) | 8.03 (6.27 - 9.79) | - |
| Sixth (n = 23) | 0.87 (0.74 - 1.00) | 0.70 (0.53 - 0.91) | 0.57 (0.40 - 0.81) | 0.47 (0.28 - 0.78) | 5.95 (4.30 - 7.59) | - |
| Overall (n = 131) | 0.87 (0.82 - 0.93) | 0.73 (0.65 - 0.81) | 0.57 (0.49 - 0.67) | 0.47 (0.39 - 0.58) | 6.12 (5.85 - 7.39) | 6.41 (4.52 - 8.30) |
The Mean/Median Survival Time (Year) and 1-, 3-, 5-, and 7-Year Survival Rate of Chondrosarcoma Patients by Variable Levels
5. Discussion
Our findings showed that the ASIR of juxtacortical chondrosarcoma, myxoid chondrosarcoma, mesenchymal chondrosarcoma, clear cell chondrosarcoma, and dedifferentiated chondrosarcoma was less than 0.1 per million person-years. However, the ASIR of conventional chondrosarcoma was higher than 1 per million person-years. Overall, males had significantly higher ASIR comparing the 95% CI of both sexes. The crude incidence rate for patients older than 40 years was higher than 2 per million person-years; however, the figure was less than 1 per million among patients younger than 20 years. Patients with chondrosarcoma of limb bones had better survival compared to other areas. Furthermore, males were more susceptible to this cancer and the elderly were also at a higher risk of death.
The data quality of the current study was evaluated by INCR and the authors. The validity of data was verified by evaluating the method of diagnosis and deleting unreliable cases. Accordingly, the chondrosarcoma was diagnosed based on the pathological findings. A population-based study in Iran may have a degree of deficiency; however, the completeness of population-based cancer data in Iran was verified by INCR published in 2016 (23). In this study, there is no claim on the completeness of the data used in survival analysis. In this case, data were obtained by telephone interview and were not complete. Though in most countries with poor registry systems, the same problem is observed and the relative survival analysis (as the best method for analyzing survival in a population-based study (28)) could not be used. The relative survival analysis requires the population life table matched to the cancer population by age, sex, race, and other risk factors. However, this information often was not available for countries with poor cancer registries and cause-specific survival could be an alternative method for relative survival analysis (29).
The only study on the prevalence of musculoskeletal tumors in Iran was conducted by Solooki et al. in Shiraz. Chondrosarcoma was the third most prevalent malignant tumor in their study following osteosarcoma and Ewing’s sarcoma (30). In another study based on INCR data, the ASIR of osteosarcoma was found to be 1.25 in 1 million (22), lower than the ASIR of chondrosarcoma in our study. There may be a change in the trend of chondrosarcoma and Ewing’s sarcoma in Iran, suggesting a rise in the number of patients with chondrosarcoma. Such a trend is also seen in England, where ASIR of chondrosarcoma has risen from 1.7 to 2 per million people (31). The same was seen in Netherland as chondrosarcoma incidence has increased over the years (6). In contrast to Ewing’s sarcoma, the incidence of chondrosarcoma increases in older adults (32). An increase in life expectancy and aging may contribute to an increase in chondrosarcoma incidence among the Iranian population.
ASIR of chondrosarcoma varies between 1 and 3 per million people in different countries (33). It is 1.2 in Taiwan (34), 2 in England (31), 2.85 in Norway (35), and 8.78 in the Netherlands (6). ASIR is much lower in our study compared to that of studies in developed countries. There are several reasons for this difference. Firstly, higher life expectancy in these countries results in more aged people in these countries. So, as chondrosarcoma’s incidence increases with age, the total incidence of chondrosarcoma will be higher in developed countries. Secondly, greater resources and better diagnostic tools can lead to the detection of chondrosarcoma in more patients and a lower incidence of chondrosarcoma in Iran may be due to undiagnosed patients. Thirdly, distributions of cancers’ risk factors are not the same in developing and developed countries, which can lead to difference in the prevalence of the disease in different countries.
Conventional was the most prevalent morphological subtype in our study (84.7% of patients with chondrosarcoma). This finding is in line with previous studies as the conventional subtype was the more prevalent subtype in studies Netherland and the USA (6, 20). The prevalence of other chondrosarcoma subtypes in our study is more comparable with Guiffrida et al.’s findings in the USA, where myxoid and mesenchymal subtypes were respectively the second and third most prevalent subtypes in their study (20). In the study of van Praag Veroniek et al. in the Netherlands, the dedifferentiated subtype, which leads to higher mortality was the second most prevalent subtype (6).
The ASIR of chondrosarcoma was slightly higher in males (1.73 in 1 million people) than females (1.27 in 1 million people). This finding is supported by previous studies in Norway (35), the USA (20), and Taiwan (34). In general, it seems that the incidence of chondrosarcoma is a bit higher in males. We did not find a male-dominant pattern for clear cell and mesenchymal chondrosarcoma, which may be due to the small sample size of these subtypes in our study. In this regard, in previous studies on larger sample sizes, male predominance was reported for these subtypes, too (36, 37).
We found that the age-specific incidence rate of chondrosarcoma and conventional and myxoid subtypes increases with age, and most patients are between 20 and 59 years old. The only exception is mesenchymal chondrosarcoma, which has the highest age-specific incidence rate in the second and third decades of life and decreases gradually. Our finding is in line with previous studies as the age-specific incidence rate of chondrosarcoma was demonstrated to increase significantly with age, especially those affecting long bones of the lower limb and central axis (32, 34). This pattern is different from other malignant bone tumors such as Ewing’s sarcoma and osteosarcoma, which are more prevalent in people who are younger than 25 years (32, 34).
The mean age of patients in our study was 44.08 at the time of diagnosis, which is considerably less compared to studies in Norway, the USA, and the Netherlands, wherein the mean age at the time of diagnosis was above 50 years (6, 20, 35). We found that patients’ survival rate decreases significantly in those in the 6th decade of their life. This result is in line with the results of Guiffrida et al., who found that the survival rate decreases significantly in those who are 50 years or older (20). Although our patients are younger than in other countries, the 5-year survival rate is 0.57 in our study, which is slightly less than in other countries (20, 31). The chondrosarcoma stage at the time of the diagnosis and its size affect the survival rate of patients. Delay in diagnosing patients with chondrosarcoma in Iran can be a reason for less survival of patients in Iran despite their younger age. We used data from 131 patients for survival evaluation, where 69 (52.67%) patients had a C-code and 41 had chondrosarcoma in areas other than limbs. In other studies, chondrosarcoma was more prevalent in limbs (6, 20, 31). Axial lesions have a worse prognosis compared to appendicular lesions (20). We also found that those with a C-code of 40 had a better survival rate (5-year survival rare = 0.62) than those with a C-code of 41 (5-year survival rate = 0.53). A higher prevalence of axial lesions in Iranian patients may be another reason for worse survival rates despite being younger at the time of diagnosis. Higher mortality of chondrosarcoma in Iran indicates a need for better case-finding and follow-up of patients with chondrosarcoma in Iran as timely diagnosis and proper care may improve the patient's outcomes and survival. Specialized musculoskeletal tumor registries may also be beneficial for a more comprehensive evaluation of musculoskeletal tumors, including their management and survival for future interventions (38).
5.1. Limitations
Our study was of retrospective cohort type, which led to missing some data of patients. This was more prominent for evaluating the survival rate of patients. We did not have the outcome of most patients; thus, it can affect our analyses. We also did not have information regarding the grade, stage, and chondrosarcoma size, which significantly affect the survival rate. More comprehensive studies on patients with chondrosarcoma can be useful in the future for better assessment of chondrosarcoma survival in Iran.
5.2. Conclusions
This is the first national study on chondrosarcoma in Iran, which gave us a good insight into its incidence rate and survival. There are similarities between Iran and other countries regarding the prevalence of chondrosarcoma subtypes and their prevalence among males and females. The incidence of chondrosarcoma in Iran is not as high as in other countries but as patients are younger in Iran, the survival rate is worse compared to other countries. Hence, better case findings and better care are needed to improve the outcome for patients in Iran.