1. Background
Radiation therapy (RT) aims to control cancer using radiation while reducing the complication of normal vital organs to the minimum or even zero. Recently, new methods have been introduced in treatment planning systems to provide more biological indicators for a treatment plan including tumor control probability (TCP) and normal tissue complications probability (NTCP) (1-3).
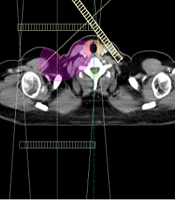
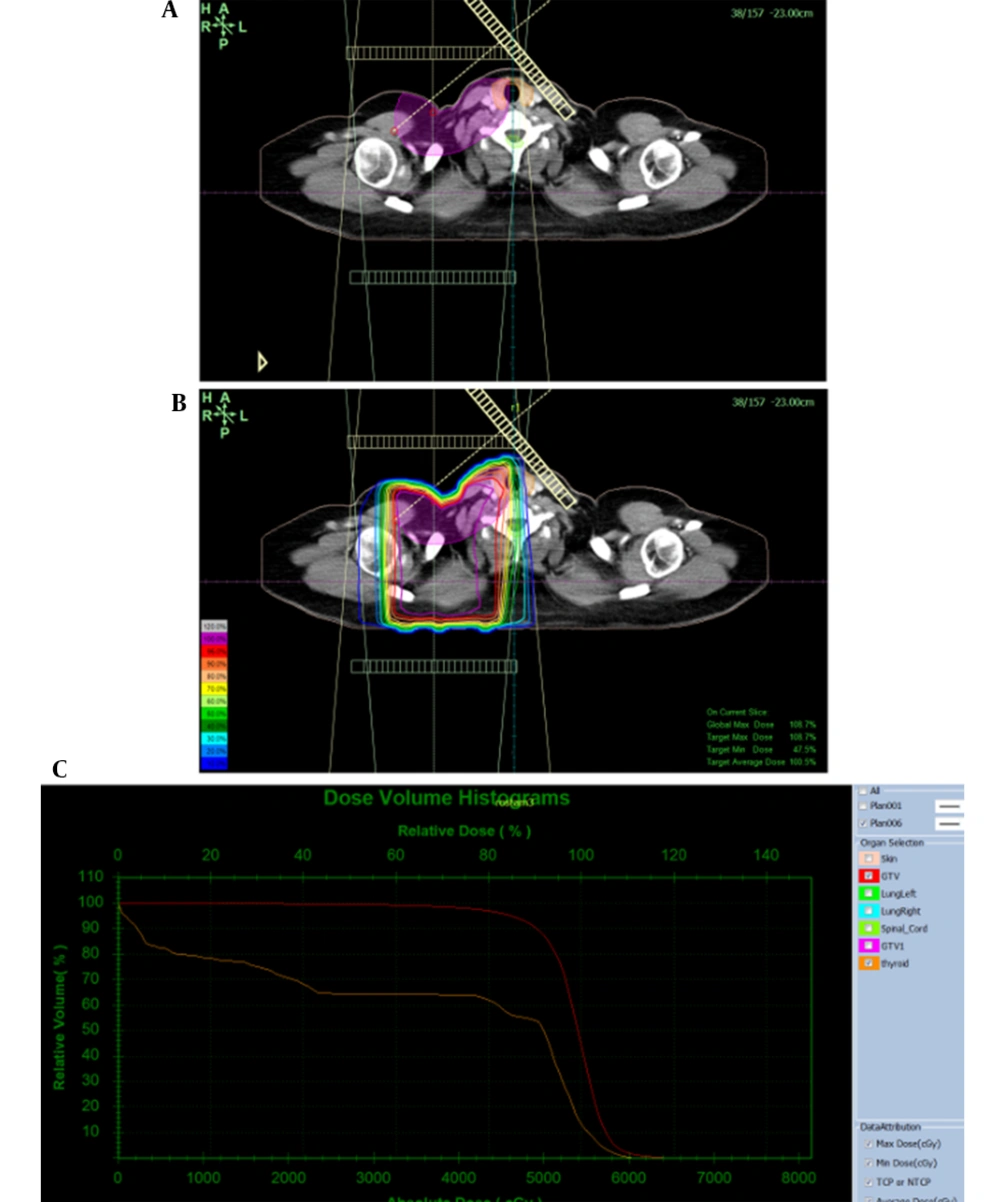
Surgery, RT, and systemic therapy are all standard and routine treatments for breast cancer depending on the stage and clinical presentation (4). In the radiotherapy changes in the local treatment of patients with breast cancer who are at high risk of locoregional recurrence could affect the risk of recurrence significantly, and this might potentially have an impact on the long-term mortality. However, in cancer survivors, RT-induced toxicities to adjacent normal tissues might cause severe disease (5). RT-induced thyroid dysfunction in breast cancer survivors who received supraclavicular (SCV) area RT is commonly overlooked (6), while a part of their thyroid gland is irradiated (Figure 1).
According to previous investigations, primary hypothyroidism (HT) is one of the most common, late-start radiation effects of the thyroid gland (7). This complication can be diagnosed in clinical and subclinical forms. Clinical HT is determined by low free thyroxin (FT4) and high thyroid-stimulating hormone (TSH). While in subclinical HT normal FT4 and high TSH are observed. Subclinical HT is the most frequent kind of thyroid dysfunction caused by radiation, followed by clinical HT, and also it can result in clinical symptoms such as hypercholesterolemia and accelerated atherosclerosis (6). For clinical HT, multiple clinical symptoms have been reported. Some of them are slowed mentation, depression, chronic fatigue, skin dryness, muscle aches, and forgetfulness (7, 8). According to some reports, radiation-induced hypothyroidism (RHT) has been reported from a minimum of 3 months to a maximum of 20 years after radiotherapy and with an average of 1.4 to 1.8 years (8, 9). The exact tolerance dose of the thyroid gland has not been identified (6). However, it has been estimated that with standard fractionation, the absorbed dose critical for the incidence of RHT varies between 26 and 40 Gy (10, 11).
In a study, Bakhshandeh et al. (12) estimated the D50 parameter for incidence of HT using 6 different radiobiological models, and approximately a dose of 44 Gy was reported for the D50 value. The implemented NTCP models showed a parallel architecture for the thyroid gland. Akyurek (6) reported a median time of 9 months for the development of RHT with a median follow-up time of 25 months. They also concluded that irradiation of the SCV region of patients with breast cancer increases the risk of HT after radiation treatment.
Studies in recent years have focused on estimating model parameters and NTCP for various important organs such as the thyroid (13). Recent modeling studies have helped to predict the dysfunction of the thyroid gland after RT, however, in most of the previous studies, the entire thyroid gland was irradiated as an endangered organ (6, 13), and a few studies have been conducted on the partial thyroid irradiation in breast cancer treatments.
2. Objectives
This study was undertaken to investigate the predictive performance of LKB and Log-Logistic models for radiation-induced thyroid dysfunction in patients with breast cancer. Another goal of the current research was to estimate the best-fit parameters for the above-mentioned NTCP models in patients with SCV radiation treatments.
3. Methods
3.1. Patients
In this research, 52 patients with breast cancer who received SCV irradiation in Madani and Valiasr hospitals (Tabriz, Iran) were studied. The median age of the patients was 48 years (range, 25 - 79 years). The median prescription dose to planning target volume was 50 Gy (range, 42.5 - 55 Gy). Patients with primary thyroid disease and thyroid surgery were excluded from the study. The study was prospective sequentially enrolled research in which the patients were informed about the research purposes and consented to participate in the study. Patients with a normal primary thyroid test were included in the study. Patients’ thyroid blood tests before radiotherapy were considered as a basis for evaluating the next blood test results. The research was accredited by the ethic committee of Tabriz University of Medical Sciences and the health ministry of Iran (Ethics code: IR.TBZMED.REC.1398.1039).
3.2. Treatment Planning
In this prospective study, patients were treated with an Oncor (Siemens, Germany) linear accelerator using 6MV photon beams and an isocentric technique in 25 fractions. Three-dimensional conformal (3DC) treatment planning was performed based on CT scan images of patients and a TiGRT treatment planning system, version: 1.0.10.582 (Linatech, Sunnyvale, CA, USA). For breast treatment, clinical tumor volume included tumors and axillaries for which doses of 5500, 5250, 550, 4500, and 4250 cGy were prescribed. For delineation of the planning target volume, a 5 mm margin was given to the CTV. The plans consisted of 2 medial and lateral tangential beams for the chest wall or breast and 2 anterior-posterior parallel opposed beams for the SCV region.
3DCRT treatment plans were prepared and confirmed by the research team including supervisors and the thesis author. From these plans, thyroid dose-volume histograms (DVHs) were extracted in a differential format as absolute doses and relative volumes for all patients. To ensure comparable results, dose per fraction and total dose were converted to the equivalent dose of 2 Gy per session using the linear-quadratic formula with α/β = 3 Gy.
3.3. Endpoint Definition
The time to endpoint (HT incidence) was determined from the date of treatment completion to the observation of the HT.
Before starting RT, to have baseline data, blood samples were taken from patients to specify the levels of TSH (normal range, 0.27 - 5 mIU/L), T3 (normal range, 0.7 - 2 ng/mL), T4 (normal range, 5.1 - 14.1 μg/dL), FT3 (normal range, 3.1 - 6.8 pmol/L), and FT4 (normal range, 12 - 22 pmol/L). Then, to monitor the function of the thyroid gland for all patients after RT, a thyroid test was taken from each patient 4 times after RT (once every 3 months) and the changes related to the level of TSH hormones in post-radiation tests were compared to baseline data.
During this study, the incidence of HT was considered as a combination of subclinical HT and clinical HT. Accordingly, patients with low FT4, high TSH, or normal FT4, and high TSH were considered RHT.
3.4. NTCP Models
Thyroid dose-response modeling was performed using two NTCP models including LKB and log-logistic based on the generalized EUD concept due to their simplicity or widespread use (14). For both models, DVHs of patients were reduced to gEUD values considering the non-uniformity of absorbed dose in the irradiated thyroid gland. The value of parameters for radiobiological models has been extracted from clinical data in this study. For the thyroid gland, the α/β ratio of 3 Gy was used (12).
The Lyman-Burman-Kutcher (LKB) model was primarily proposed to estimate the complications for uniformly irradiated organs (15). The LKB model has several parameters including D50, n, and m. The LKB model was formulated using the probit equation and employs the power-law method of Mohan et al. (16). The model is presented in Equations 1 – 3.
The parameter of Deff stands for the effective dose, D50 is the uniform organ dose that leads to 50% complications, m symbolizes the slope of the sigmoid curve, n stands for the volume effect, and Vi is the fraction of organ volume with a dose bin of Di.√2π.
3.4.1. The Log-Logistic Model
This model is based on a logistic mathematical function to estimate the dose-response curve for different organs irradiated in RT (17). For absorbed dose values, it employs the concept of "generalized equivalent uniform dose" (gEUD) which was initially used to describe tumors, but it was gradually extended to include both tumors and normal tissues (18). The model is presented in Equation 4.
Where the γ50 stands for the steepness of the dose‑response curve. Other parameters are the same as the LKB model which was explained earlier.
3.5. Statistical Analysis
In this study, the Bayesian method was used to estimate the parameters (a, n, m, TD50, and γ50) used in radiobiological models. Bayesian statistics is one of two main perspectives in statistical science along with the frequentist approach. Bayesian statistics has its features and, in contrast to the frequentist approach, gives estimates on probabilities of unknown parameters. This uncertainty has arisen from the imperfect knowledge of the researcher about the parameter distribution. Bayesian methods combine the researcher's belief or previous knowledge on the parameter distribution, called prior distribution, with available observed data with presumed distribution to provide updated knowledge on the parameter distribution, called the posterior distribution. This would be helpful especially when the current data contains limited information due to the small sample size. Then, desired information such as the mean and SD of the posterior distribution is calculated. The Markov chain Monte Carlo (MCMC) methods are employed for the simulation of the posterior distribution. The application of Bayesian statistics has become popular since the development of the MCMC methods which enables researchers to fit complex models that may be computationally expensive or even impossible to fit (19). We fitted the models with improper priors to remove the impact of prior knowledge on our estimates and just benefited from the fitting facilities of the Bayesian method. The fitting of the models was performed using Stan software in 2.3.3 R software (20). Model fit was assessed using a fully Bayesian WAIC criterion, smaller values of which indicate better fit.
4. Results
Table 1 provides an overview of the patients' demographic, clinical, and treatment properties.
| Characteristic | No. of Patients |
|---|---|
| Sex | |
| Female | 52 |
| Age (y) | |
| Mean | 48.75 |
| Median | 48 |
| Range | 25 - 79 |
| Mean dose of thyroid (cGy) | |
| Minimum | 23.1827 |
| Maximum | 5072.079 |
| Mean | 1824.609 ± 673.78 |
| Cancer site | |
| Right breast | 26 |
| Left breast | 26 |
| Bilateral | 0 |
| Volume of thyroid (mL), mean ± SD | 17.85 ± 8.91 |
The Data of Demographic and Clinical Features of the Studied Patients
Twenty-one (40%) out of 52 patients with breast cancer developed RHT at a follow-up of 1 year after the end of radiation treatment. Patients with HT had a median duration to the endpoint of 6.7 months.
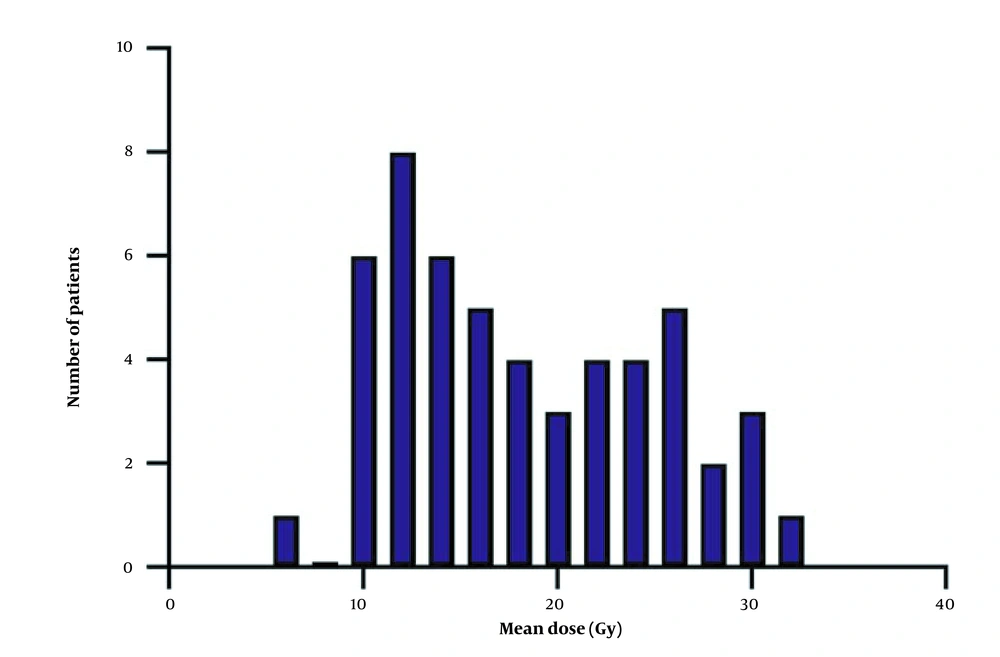
The mean dose to the thyroid gland in the patients was 18.24 Gy. For all studied patients, a mean volume of the thyroid gland of 17.86 mL was obtained.
In this study, the effect of chemotherapy was not investigated because most patients with breast cancer were first treated with chemotherapy and then with radiotherapy.
Figure 2 shows the distribution of the mean thyroid dose received for all patients. Besides, radiation and clinical data for total patients are provided in Appendix 1.
4.1. NTCP Models
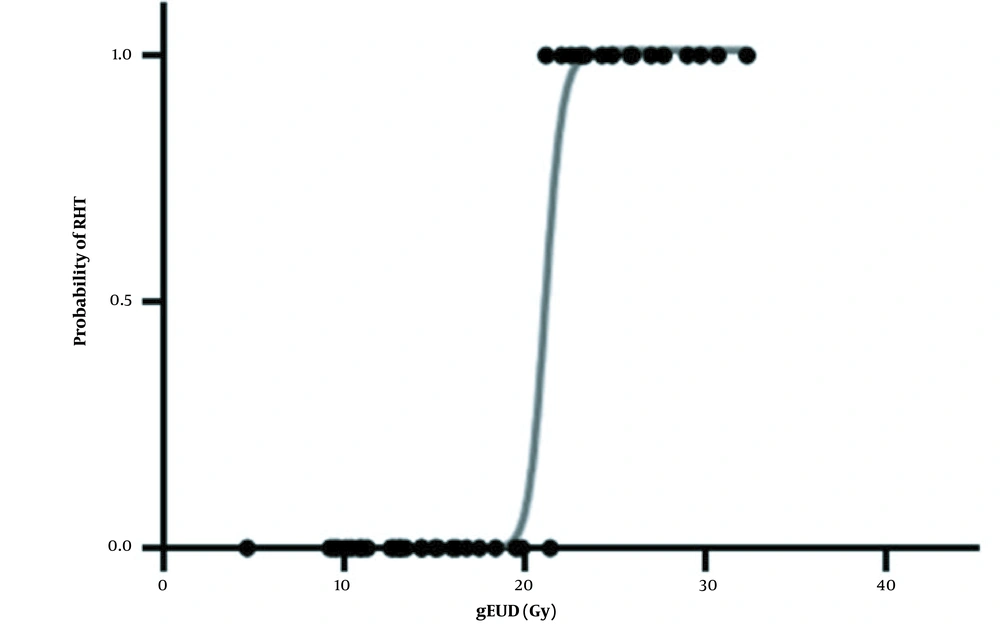
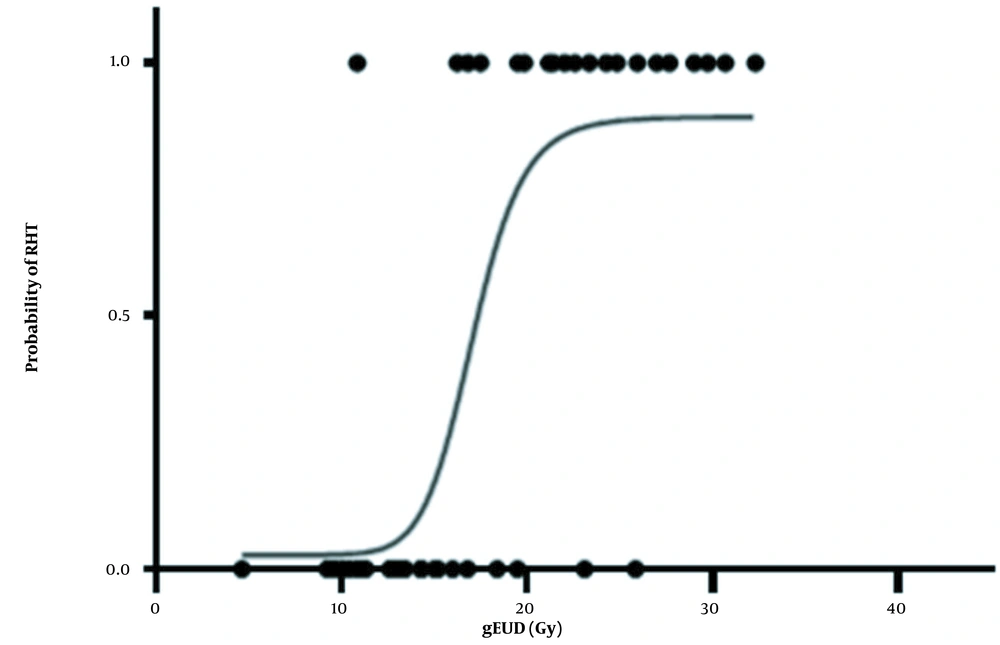
The optimum values (with 95% CIs) for studied parameters are tabulated in Table 2. The model parameter D50 appears in both NTCP models. For the LKB and Log-Logistic models, the fitted values of D50 were 37.71 and 25.50 Gy, respectively. For the LKB model, the value of m = 3.52 was obtained. Also, the values of the LKB model's parameter of n revealed a parallel architecture for the thyroid gland. In Figures 3 and 4, NTCP curves of the LKB and Log-Logistic models and ncidence of RHT as a function of gEUD are observed. The continuous linear curve was obtained using the best-estimated parameters. The curves for both models were plotted separately. Different dose-response behaviors were observed for the models, but overall, with an increase in absorbed dose the incidence of HT increased. The dose-response curves exhibited sigmoid shapes for the LKB (Figure 3) and log‑logistic (Figure 4) models. Also, the slope of the log-logistic model curve was higher than that of the LKB model.
| Model and Parameters | Values | %95 CI |
|---|---|---|
| LKB | ||
| n | 0.14 | 0.017 - 0.336 |
| m | 3.52 | 2.415 - 4.791 |
| D50 | 37.71 | 30.553 - 45.839 |
| Log-Logistic using gEUD | ||
| a | 1.093 | 0.196 - 2.474 |
| γ50 | 0.017 | 0.008 - 0.061 |
| D50 | 25.500 | 15.983 - 35.362 |
Model Parameters with 95% CI and Goodness of Fit Values of the Models for Hypothyroidism Endpoint
The results of fitting clinical data with LKB and logistic-Log models for all patients are given in Table 3. These results include parameter estimation with 95% confidence in WAIC criteria. The lower the WAIC value for a model, the more consistent the clinical data. In other words, the lower the WAIC criterion, the better the model performance compared to the clinical data. Furthermore, the highest-ranking for the Log-Logistic model was shown by the WAIC values, and then, the LKB model ranked as the next model.
| Models | WAIC |
|---|---|
| L-K-B | 86.7 ± 6 |
| Log-Logistic | 73.99 ± 1.2 |
Model Ranking Based on the WAIC
5. Discussion
This study aimed at estimating the NTCP of the partially irradiated thyroid gland after RT for patients with breast cancer. The clinical follow-up data of the patients for HT was recorded and analyzed. Then, 2 analytical models were employed for NTCP calculations of RHT and their predictions were compared with the clinical data. Also, the parameters of the LKB and Log-Logistic models were estimated for RHT and their predicting performances were ranked in comparison to observed clinical outcomes.
Even though most researchers have focused on RHT in patients with head and neck cancers or lymphoma, evidence suggests that it has happened in patients with breast cancer who have undergone RT to the SCV initially emerged in the 1980s (21). Subsequently, several studies revealed that irradiated patients, especially those exposed to the SCV region, are associated with a higher prevalence of RHT (4, 22).
Considering both clinical and subclinical RHT as endpoints, the D50 of approximately 32 Gy was obtained. The parameters that were estimated using clinical data or the LKB model were n = 0.14, m = 3.52, and D50 = 37.71. For the Log-Logistic model the parameters of γ50 = 0.0175, D50 = 25.50, and a = 1.093 were estimated for the studied patients.
Also, the results were compared to previous studies in which the thyroid gland was completely irradiated. It should be noticed that there was no comparative study in which radiobiological models have been examined for the partially irradiated thyroid glands.
Comparing our results with previous studies, Bakhshandeh et al. reported the D50 of approximately 44 Gy, with any HT in the patients at 1 year was regarded as the endpoint. In their study, the fitted parameters for the LKB model of n = 0.92, m = 0.25, and D50 = 44.3 Gy were reported (12). Although, those values represented the situation where the entire thyroid gland was irradiated as an organ at risk, in the current study, only a part of the thyroid gland volume was irradiated and considered for the main analysis. Consequently, the D50 values obtained from the LKB models were slightly different between the two studies.
According to the study by Jereczek-Fossa et al., the effect of chemotherapy and endocrine therapies on the incidence of HT is a controversial subject (7). However, other research suggested that adding chemotherapy to the treatment process does not raise the chance of developing HT (12, 23). In our study, the effect of chemotherapy was not investigated because all the patients with breast cancer were first treated with chemotherapy and then with radiotherapy.
Our findings can be compared with the other studies such as Huang et al. in which they found that 32% of breast cancer patients with SCV radiation developed RHT with a 5-year follow-up. The mean dose of the thyroid gland in their patients treated with 3D-CRT was 17.41 Gy (4). Compared to their data, our study also found that 40% of patients developed RHT and the mean dose to the thyroid gland in the patients was reported as 18.24 Gy. Consequently, the results of our study are consistent with the findings of Huang et al. and compared to the general population, wich showed a notable rise in the incidence of HT. In another study by Akyurek (6) that conducted on thyroid complications in patients with SCV irradiation, individuals who received 50 - 60 Gy had a 21% chance of developing HT. Their reported incidence rate was lower than our rate of 40%. They also analyzed the mean dose, volume, and partial volumes of V10-50 for thyroid according to the received DVHs. It was shown that V20 (P = 0.05), V30 (P = 0.05), and V40 (P = 0.02) and mean thyroid dose ≥ 36 Gy (P = 0.05) had a considerable influence on the development of HT, whereas thyroid volume was not linked to the development of HT (P = 0.99).
Following supraclavicular RT, we noticed a significant rise in the development of HT in women with breast cancer. According to our findings, the major changes in thyroid function occurred between 3 to 6 months following RT. Our results showed that according to the WAIC index, Log-Logistic is the most suitable model. However, the LKB model satisfactorily performed and was also ranked as the second model.
5.1. Conclusions
In the current study, the effect of partial irradiation of the thyroid gland on the incidence of HT was analyzed. A significant incidence (40%) of HT occurred among patients with breast cancer after the completion of RT. According to the WAIC index, the highest-ranking for the Log-Logistic model was shown, and then, the LKB model ranked as the next model. However, the NTCP results of 2 radiobiological models, LKB and Log-Logistic in estimating the incidence of RHT in patients with breast cancer were very close.
Finally, the application of the Log-Logistic EUD modeling for the NTCP estimation of thyroid complications following RT of the SCV region is recommended.