1. Background
The anticancer drug doxorubicin (DOX) is mentioned to exhibit a choice treating effect on many malignancies, especially hematological ones, but the liver, blood vessels, kidneys, and other organs could be harmfully hurt by the administration of this drug (1). The most vulnerable organ to DOX damage is the liver due to its main metabolic activity. DOX is proved to induce oxygen radicals such as superoxide anions, hydrogen peroxide, and hydroxyl radicals. DOX can inactivate superoxide dismutase and reduce glutathione peroxidase. Then, the high rate of radicals of the oxygen species (ROS) production is compared to antioxidants, the high rate of tissue destruction. It is proved that the ROS derived from DOX and its metabolites in the liver can start extrinsic and intrinsic apoptotic pathways with receptor and caspase three activations respectively. Then, DOX can induce apoptosis in addition to necrosis of hepatocytes (2). The fact of its toxic effects on the liver and other organs is the harmful character that leads to use with caution. Then, it is necessary to supplement an antioxidant with doxorubicin to reduce its side effects.
The melatonin from the pineal gland is described as a factor that changes and regulates the sleep-wake cycle, circulatory system, neuroendocrine, and immunity against pathogens (3). Melatonin can behave as an anti-inflammatory and antioxidant agent. Melatonin has a direct neutralizing effect on ROS. It can induce the synthesis of the other antioxidants and help to store them. Melatonin has a potent ability to react and inactivate about 10 radical species. In contrast, other antioxidants can inactivate only one radical molecule (4). The extracts of many plants have been demonstrated as a source for preventing hepatic lesions. Cichorium intybus (C. intybus), a plant with hydroxycinnamic acids and flavonoids as its dominating compounds, helps heal the hepatic injury. Administration of C. intybus can increase the number of beneficial flora in the intestine (5, 6). Administration of C. intybus root extract also improves blood flow through circulation because of its inhibitory effect on the angiotensin-converting enzyme. It is known as a treatment for muscle aches and influenza (7, 8). Many studies have shown that C. intybus could improve the hepatic lesions induced by pathogens or drugs realizing ROS (8, 9). Administration of the C. intybus can reduce the territory effect of the ischemic reperfusion injury of myocardia. This is due to the induction of chemokine receptor type 4 and antioxidant ability (10). A study about lead acetate testis toxicity in male rats proved that C. intybus leaf extract can prevent testicular lesions (11). Tumor necrosis factor α (TNFα) is a cytokine with the ability to induce death signals in addition to other inflammatory functions. In the liver, TNF-α is important for regeneration, proliferation, and fibrosis, but can induce hepatitis in conditions such as viral hepatitis or fatty liver diseases. TNFα is a cytokine with the ability to induce death signals in addition to inflammatory functions. TNF-α is significant for hepatic regeneration, proliferation, and fibrosis. It can start hepatitis in the condition such as viral hepatitis or fatty liver diseases. TNF-α has two different roles in the liver, one is as a factor of cell death and the other is a hepatocyte and fibroblast proliferator (12). 20-hydroxyeicosatetraenoic acid is the substance of a pro-inflammatory metabolite of the arachidonic acid. According to a study, acute DOX toxicity could increase the synthesis of the 20-hydroxyeicosatetraenoic acid in the liver and kidney. DOX alters the function of P450 and Epoxide hydrolase enzymes. Then, DOX would increase inflammation (13). Some reports defined the relationship between TNFα and vast lesions of the liver or hepatotoxicity. In microbial septicemia, principally when LPS or endotoxins are released, this cytokine is excreted by Kupffer cells. TNFα is the reason for hepatocyte injury (14, 15). Almost all anticancer drugs such as cisplatin and DOX can develop hepatotoxicity. They, then, elevate related enzymes including ALT, AST, GGT, ALP, and total bilirubin. Some investigators proved that antioxidants and plant extracts could be a benefit for reducing hepatic lesions (16, 17). The histopathological examination has a powerful ability to determine the range of the toxic lesions in all organs (18).
2. Objectives
Cichorium intybus (C. intybus) is a plant with hepatoprotective effects. Melatonin is an antioxidant similar to vitamins. We investigated the repairing effects of C. intybus -melatonin together against doxorubicin-induced hepatotoxicity.
3. Methods
3.1. Plant Materials
C. intybus (whole Plant) with intact appearance picked up in May 2021. A botanist from Biological Sciences Department identified the plants. The plants were washed and put at a 30-degree temperature to be dried. After drying, the whole plant was pulverized into a powder shape by a laboratory mill and put at -20°C until the accumulation (8).
3.2. Preparation of Extract
3.2.1. Preparation of Aqueous-alcoholic Extract
The whole plant was powdered and taken equally and extracted by maceration procedure with alcohol (95% Ethanol) and water for 7 days in an occasional shaking and stirring flask. We filtered the extract, concentrated it, and then dried it by evaporation. The percentage of alcoholic extract of the whole plant was 18% w/w. We stored the extract at -4°C (7).
3.2.2. Preparation of Dried Extracts and Standard
We macerated 100 g of the whole plant dry C. intybus in 1 liter of methanol for 24 hours, filtrated, and the prim extract was collected. In the second stage, under reflux in a water bath, we poured 1 liter of methanol on the sample residue, boiled it for 2 hours, and filtered it. The filtrate was added to the previous crude extract. Next, we added 1 liter of distilled water to the residue. We put it leftover nightly in the room and then filtered it. The filtrate was added to the previous crude extract. Another 1 liter of distilled water was added to the residue, boiled for 2 hours, and filtered. The hot water filtrate was added to the previously collected extract. This manner belongs to the hydroalcoholic extract. There we dried the hydroalcoholic extract at 60°C and froze it until usage (19).
3.3. Animal Treatments
The present study was performed on 30 balb/c mice in the weight range of 20 to 25 g. Before starting the study, to relieve stress and ensure the health of the mice, all of them were kept in cages for 14 days and fed the same diet during storage. After clinical health assessment, the studied mice were randomly divided into 5 experimental groups of 6 animals each. The groups included control (saline group); mice were orally administered with normal saline (1 mL/kg) for 10 days; DOX; DOX (Cell Pharma, 50 mg per 25 mL), was injected at a dose of 15 mg/kg intraperitoneal (20). Chicory; mice were daily administered with chicory whole plant extract, 500 mg/kg (21), for 10 days followed by one dose DOX injection at 15 mg/kg intraperitoneal. Melatonin; mice were daily administered with melatonin, 10 mg/kg (22), for 10 days followed by one dose DOX injection at 15 mg/kg intraperitoneal. Both Chicory-Melatonin; mice were daily administered with both 500 mg/kg chicory and 10 mg/kg melatonin, for 10 days followed by one dose of DOX injection at 15 mg/kg intraperitoneal. Our curing and treating behavior of animals was according to Legislation for the protection of animals used for scientific purposes.
3.4. Biochemical Parameters
On day 20, we took blood samples from the cervical vein, weighted mice accurately, and then euthanized them ethically. Serum Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Gamma-glutamyl transferase (GGT), Alkaline phosphatase (ALP), and total bilirubin were measured by using an automatic biochemical analyzer (Accent 200, China). These measures are indicators of liver (hepatotoxicity) lesions (14).
3.5. Histopathological Sample Preparation and Evaluation
After each necropsy, we put the livers in 10% formalin buffer for histopathological and immunohistochemical evaluation. A histopathological score of hepatic tissues was performed as 0 = absent; 1 = low or weak; 2 = mild; 3 = moderate; and 4 = high or frequent, and the total score was the basis of judgment (23).
3.6. Immunohistochemical Sample Preparation
The anti-TNFα antibodies were used in this study to prove the degree of the inflammation. Avidin-biotin peroxidase immunohistochemical method (ABC) was used to detect the TNFα positive areas in the livers of all experimental groups. We deparaffinized the 5-µm sections exposed them to TNFα monoclonal antibody (dilution 1:100; DAKO), and then mounted them. Positive and negative controls evaluated the staining procedure. The pathologist described the lesions in a high-power field for all slides and, then, captured them by digital camera. Five high power fields were observed at random.
3.7. Statistical Analyses
First, the data obtained from the study were presented as the mean ± SD and, then, analyzed by SPSS 22, using the one-way analysis of variance (ANOVA) followed by the Bonferroni test for parametric data and the Kruskal–Wallis one-way analysis of variance test for non-parametric data. P-values less than 0.05 were considered statistically significant.
4. Results
4.1. Toxicity
All mice that received C. intybus extract or melatonin lived with normal weight and body condition during the study. In contrast to the DOX-treated mice, this drug initiated many side effects such as tangled body hair, poor body condition, loss of activity, and weakness.
4.2. Biochemical Parameters
The automatic cell counter analyzed the ALT, AST, GGT, and ALP activities in addition to total bilirubin, higher despite the non-significantly in the DOX group than in the other groups. There was no significant (P < 0.05) relationship between treatments (Table 1).
| Groups | AST (unit/L) | ALT (unit/L) | GGT (unit/L) | ALP (unit/L) | Total Bilirubin (mg/dL) |
|---|---|---|---|---|---|
| Control (saline) | |||||
| Mean ± SD | 215.75 ± 100.20 A | 80.50 ± 14.27 A | 0.25 ± 0.50 A | 225.75 ± 141.54 A | 19.25 ± 8.53 B |
| %CV | 46.44 | 17.72 | 2.0 | 62.69 | 44.31 |
| DOX | |||||
| Mean ± SD | 221.00 ± 65.84 B | 102.00 ± 76.63 B | 1.17 ± 1.94 B | 307.67 ± 33.90 B | 15.17 ± 5.15 B |
| %CV | 29.79 | 75.12 | 1.65 | 11.01 | 33.94 |
| Chicory | |||||
| Mean ± SD | 150.80 ± 24.50 B | 62.40 ± 6.46 A | 0.40 ± 0.54 A | 214.2 ± 117.69 A | 32.80 ± 23.59 A |
| %CV | 16.24 | 10.35 | 1.35 | 54.94 | 71.92 |
| Melatonin | |||||
| Mean ± SD | 49.67 ± 17.94 B | 129.33 ± 18.03 A | 0.50 ± 0.54 A | 184.83 ± 95.64 A | 16.0 ± 3.16 B |
| %CV | 36.11 | 13.94 | 1.08 | 51.74 | 19.75 |
| Both (DOX + chicory / melatonin) | |||||
| Mean ± SD | 67.60 ± 11.86 B | 125.67 ± 10.78 A | 0.60 ± 0.54 A | 254.6 ± 61.35 A | 11.5 ± 1.73 B |
| %CV | 8.57 | 8.58 | 0.9 | 24.09 | 15.04 |
a Analysis was done by Kruskal-Wallis for AST, GGT, and total bilirubin and one-way analysis of variance test for ALT and ALP. A: Significant difference from DOX. B: Significant difference from A.
4.3. Histopathological Structure of the Liver
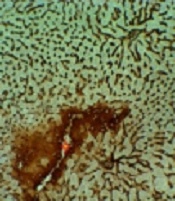
The liver section in mice of the control group had normal lobules composed of straight columns of hepatocytes that extend from the central vein to the periphery of the portal areas (Figure 1A). The liver of the DOX group showed severe lesions. These include micro and macrovesicular vacuolar degeneration, necrosis, and inflammation of the parenchyma. The necrosis was composed of nuclear pyknosis and more eosinophilia or loss of cytoplasm. All study groups were significantly better than the DOX group. It was observed in the treatment groups that melatonin compared to chicory improved the livers. The Co-administration of melatonin and chicory, the most ameliorating livers tissues (Figure 1A - E; Table 2).
Histopathological evaluation of the livers. A: Control group not exposed to doxorubicin. B: DOX group we treated with doxorubicin. C: Chicory, D: Melatonin, and E: Chicory + melatonin compared to DOX group (P < 0.05); (HE × 200). Freeform: Vacuolar degeneration; Yellow arrow: Necrotic hepatocyte; Chevron: Inflammation; P: Portal vein; V: Central vein
| Groups | Necrosis | Leucocyte Infiltration | Vacuolar Degeneration | Congested Blood Vessels | Total Score |
|---|---|---|---|---|---|
| Control (saline) | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 0.00 A |
| DOX | |||||
| Mean ± SD | 3.67 ± 0.51 B | 1.83 ± 0.75 B | 2.50 ± 1.22 B | 2.17 ± 0.75 B | 11.34 B |
| %CV | 14.10 | 41.10 | 48.80 | 34.70 | |
| Chicory | |||||
| Mean ± SD | 2.17 ± 0.75 C | 1.33 ± 0.5 B | 1.33 ± 0.51 C | 1.50 ± 0.54 B | 8.17 C |
| %CV | 34.70 | 38.70 | 38.34 | 36.50 | |
| Melatonin | |||||
| Mean ± SD | 1.67 ± 0.51 C | 0.67 ± 0.51 C | 1.00 ± 0.00 C | 0.67 ± 0.51 A | 4.18 D |
| %CV | 31.00 | 77.50 | 00.00 | 77.50 | |
| Both (DOX + chicory / melatonin) | |||||
| Mean ± SD | 0.83 ± 0.75 A | 0.33 ± 0.51 D | 1.00 ± 0.00 C | 0.33 ± 0.51 A | 2.49 D |
| %CV | 90.30 | 0.00 | 0.00 | 154.90 |
a They were treated with Chicory extract and Melatonin. Scoring was performed as 0 = absent; 1 = low or weak; 2 = mild; 3 = moderate; and 4 = high or frequent, and also the total score. Analysis was done by Kruskal-Wallis one-way analysis of variance test. A: Significant difference from DOX. B: Significant difference from A. C: Significant difference from A and B. and D: Significant difference from A, B and C.
4.4. Immunohistochemistry Analysis of TNFα in the Liver
Vast TNFα positive areas of the liver in the DOX group revealed the important role of DOX anticancer in developing inflammation and hepatotoxicity. Otherwise, all groups that received melatonin were more potable of reducing lesions than that in the chicory-treated group. The Co-administration of melatonin and chicory significantly showed the best reduction in the TNFα positive areas and inflammation (Figure 2A - E; Table 3).
| Factor | Control (Saline) | DOX | Chicory | Melatonin | Both (DOX + Chicory / Melatonin) |
|---|---|---|---|---|---|
| TNFα % | 12.26 ± 1.35 A | 17.54 ± 0.70 B | 17.27 ± 0.95 B | 14.72 ± 0.66 C | 13.59 ± 0.98 A, C |
a Data are mean ± SD, and analysis is carried out using one-way ANOVA. A: significant difference from DOX. B: Significant difference from A. C: Significant difference from A and B.
5. Discussion
DOX is a potent inducer for the production of many free radicals in the hepatic tissues. The liver cannot eliminate it by the body-stored antioxidants (24). Hepatocytes are destroyed when DOX is metabolized by hepatic enzymes rich in mitochondria (25). The phenomena that indicated hepatic injury due to DOX induction increase ROS, reduce mitochondrial oxidation cycles, chromatin malformation and degradation, and Necrosis or Apoptosis (26). We studied the effects of Chicory extract, Melatonin, and both of them as anti-inflammatory factors. We also assessed their protecting effects against mice liver injury induced by DOX.
Melatonin can be a powerful antioxidant and antiradical agent that limits the effects of free radicals such as hydroxyl and peroxyl and peroxynitrite radicals in many organs, especially in the liver (3, 27). Melatonin is also involved in reducing the ischemia-reperfusion injury. Some researchers have revealed that the endothelial cells of small vessels may be destroyed. Following this, the repair of the hepatic lesions will not heal properly in the DOX-treated groups (28). The growth of cancers has been prevented by melatonin supplementation therapeutically, in addition to its protective ability to decrease the side effects of chemotherapy (29). Many studies have shown that C. intybus could improve the hepatic lesions induced by pathogens or drugs realizing ROS (8, 9). The leaf of C. intybus extract manifested a significant protecting effect against hepatotoxicity caused by CCl4. It also reduces the serum levels of the AST, ALT, ALP, and the total bilirubin in the CCl4 treated mice (30). On the other hand, melatonin in combination with DOX can lead to decrease high serum ALT and AST levels (31). According to this experiment, co-supplementation of chicory extract (500 mg/kg) and melatonin (10 mg/kg) could reduce ALT, AST, GGT, ALP, and total bilirubin more potential than each on its own.
We suppose that the melatonin administration may promote hepatic tissues more resistance to lipid oxidation. Pre-treatment with melatonin at 10 mg/kg followed by improving effects in the liver exposed to DOX could be demonstrated by decreasing the vascular degeneration. We found this microscopic feature in the chicory group and then proposed that the C. intybus extract at 500 mg/kg may improve the severity of lipid oxidation in the liver. Melatonin and Chicory cooperate as an antioxidant, anticancer, and detoxifiers. They can inhibit ROS production and prevent cell injury by the reaction to radicals (32). According to our results, co-administration of chicory extract (500 mg/kg) and melatonin (10 mg/kg) cure hepatic injury significantly. Histological signs of DOX administration are sinusoidal congestion, hepatocyte vacuolar degeneration, and leukocytes infiltration (24). Following the pathologic effects induced by DOX, there is an increase in the expression of some growth factors that lead to the hyperplasia of bile ducts with infiltration of the inflammatory cells (33). In this experiment, the hepatic lesions in the DOX group were moderate lobular necrosis, severe vacuolar degeneration, interface and lobular inflammatory cells infiltration, and sinusoidal engorgement. We demonstrate minimal pathological findings in all treating groups compared to what was seen previously in the Dox group alone. There are differences between all groups except that the melatonin and both chicory and melatonin group did not have any significant difference in the total score (Table 2).
Cytokines are small signaling proteins that modulate leukocytes' activity in immunity and aid in the movement of immune cells to regulate cell activity, differentiation, proliferation, migration, or inflammation against pathogens (34). The first powerful cytokine that stimulates other cytokines, such as IL-1β synthesis is TNF-α, which can exaggerate the inflammatory cascade (35). The most way that DOX creates lesions in the organs such as the liver is to pull out the synthesis of pro-inflammatory cytokines followed by necrosis and degeneration.
Melatonin can reduce the serum levels of TNF- α and IL-6 in the mice. It can increase the levels of IL-10 (36). According to some studies, melatonin can improve cholestatic liver lesions induced by α-naphthyl isothiocyanate and its oxidative stress. Generally, melatonin is the benefit for improving liver disease (37-39). NOD-like receptor protein 3 (NLRP3: Inflammasome) can activate the inflammatory responses in complicated conditions such as microbial influx or necrosis due to toxins. The inflammasome also stimulates the synthesis of the IL-1β and caspase-1 in some situations such as cancer and diabetes. Melatonin diminishes inflammation by inhibiting NLRP3 synthesis. It controls some regulatory molecules, such as microRNA and Wnt/β-catenin (40). According to our results, the group receiving DOX caused a significant increase in hepatic parenchyma TNF-α levels correlated to increased leukocytes and inflammatory processes (41).