1. Background
Vulvovaginal atrophy (VVA) is a common chronic progressive condition secondary to low estrogen levels during and after menopause (1-3). The common symptoms include dryness, itching or burning sensation, dyspareunia, and bleeding after intercourse (4-6). In this condition, a low circulating estrogen level leads to a loss of collagen, adipose tissue, and water preservation capability in the vagina. The vaginal wall undergoes contracture and the folds flatten and disappear (7, 8).
Although vaginal atrophy usually occurs during menopause, it can happen in women of any age, who experience conditions associated with decreased estrogen levels including postpartum, breastfeeding, hypothalamic amenorrhea, and treatment with anti-estrogenic medication (9-11). Women who experience aggravated menopause due to bilateral oophorectomy or chemotherapeutic regimens experience more severe and early onset symptoms (12-14). About 39% of menopausal women suffer from vaginal symptoms related to menopause. Of those, 52% have reported these symptoms affecting their quality of life. Notably, these symptoms are more prevalent in menopausal induced by chemical drugs (15, 16).
Breast cancer, as the most common malignant neoplasm among women, constitutes approximately a third of all cancer cases (17, 18). In Iran, according to the most recent national database, the rate of breast cancer standardized for age was 33.21 per 100,000 and mortality was 14.2 per 100,000 with a mean age of 49.84 years (19). VVA leading to sexual dysfunctions is common among patients with breast cancer following diagnostic and therapeutic interventions (20). Although sexual dysfunction reportedly occurs in 40 to 60% of patients with breast cancer, when we ask the patients about it, 90% of patients report at least one relevant symptom (21).
VVA symptoms are often progressive and not self-limiting; therefore, exacerbation of untreated symptoms can lower the quality of life. While the vast majority of patients need treatment, only 25% seek therapy (4). Vaginal estrogen compounds, the recommended therapy for this condition, warrant endometrial evaluation when used for longer than 6 to 12 months and are associated with risks in patients with breast cancer (8, 22).
In recent decades, there has been growing interest in complementary and alternative medicine in many countries (23). Considering the similarities between the etiology and symptoms of VVA with causes and symptoms of dry uterine dystemperament and uterine, cervical, vaginal, and vulvar fissure in Persian medicine textbooks, they may be related to such diseases and can fall under the same category (24).
“Qeirooti”, oil wax in Persian, is a mixture of wax in boiling oil and is a form of traditional drug that acts similar to moisturizers (25). Persian medicine reference books consider oil wax prepared from duck or chicken tallow as an effective treatment for skin dryness and fissures in different body parts (26).
2. Objectives
Considering that topical hormone therapy, particularly for a long period, is associated with risks in patients with breast cancer and warrants endometrial evaluation (22), this study was designed to assess the effects of a chicken tallow product as a non-hormonal alternative for vaginal use on subjective symptoms of VVA in women with breast cancer.
3. Methods
3.1. Population and Sample Size
This is a before-after clinical trial on 50 menopausal BCS above 18 years of age, who were seen for follow-up in the Oncology-Radiotherapy Clinic of Shohadaye Tajrish Hospital between March and July 2020. Considering the expected difference in the itching (as one of the outcome measures) according to a previous pilot study, the sample size was calculated to be 42, which considering the lost-to-follow rate of approximately 15%, at least 50 patients entered the study.
3.2. Eligibility Criteria for Participants
The study population included patients with breast cancer, who had a complaint of subjective symptoms of VVA (itching, burning, dryness, dyspareunia) with a minimal sum score of 3, age 18 years or older, married, monogamy, menopause, history of breast cancer, no synchronous second malignancy, completion of chemotherapy and radiotherapy, history of hormonal therapy, negative pap smear over the past year, no vaginal lubricant or moisturizer use or other vaginal medications over the past month. Exclusion criteria included refusal to participate at any point of time during the study, using a vaginal lubricant or moisturizer or other vaginal medications during the course of the study, gynecological or laboratory confirmation of vaginal infection during the study, uterine bleeding, or spotting of unknown etiology, side effects including allergy to the product being studied, acute or serious disease during study, acute psychiatric or neurological conditions, or using antidepressants during the study.
3.3. Study Protocol
To assess subjective symptoms of VVA (itching, burning, dryness, and dyspareunia) in this study, VVA subjective symptom score (VVA-SSS) form was used. The symptoms were graded, using a 5-point Likert scale (0: absent, 1: mild, 2: moderate, 3: severe, 4: very severe) and the sum of the scores from 4 symptoms constituted the score for subjective symptoms of VVA.
To prepare the vaginal cream (a Persian medicine product), which contains chicken tallow oil and beeswax was obtained from Ahmadieh Traditional Medicine Clinic (Ahmadieh Salamatkade) pharmacy, Tehran University of Medical Sciences, and chicken tallow was purchased from an authorized market of protein products in Tehran. Chicken tallow was heated and after several rounds of filtration, oil was extracted. Subsequently, in a water bath of 100°C, wax was added to the oil base until it became homogeneous. The appearance stability, drug assay, PH, and microbial characteristics (including general tests for bacteria, fungi, and yeasts, and tests for specific micro-organisms including staphylococcus, salmonella, and pseudomonas) were investigated in an accelerated manner. The drug assay was performed based on oleic acid and using the Gas Chromatography Flame-ionization detection (GCFID) method. Quality control and microbial tests on product ingredients were performed at the Medicinal Plants and Drugs Research Institute of Shahid Beheshti University of Medical Sciences.
Patients with subjective symptoms of VVA as a confirmed diagnosis by pap smear over the past year or vaginal exam by a gynecologist were referred to the research team by a radiotherapist. After ensuring that the participant met the inclusion criteria, the patient was verbally asked to score her subjective symptoms of VVA. Patients were explained about the research project and written informed consent was obtained from all participants, emphasizing that they can leave the study at any point they wish. An assessment form for subjective symptoms of VVA was, then, completed. Each patient was provided with a 30-gr tube of trial medication and 6 vaginal applicators to apply 5 g every other night before bedtime for 2 weeks. After 2 weeks, the patient was revisited and an assessment form of subjective symptoms of VVA was again completed. A second tube of the trial medication and 4 vaginal applicators were, then, provided to apply 2 nights a week for the next 2 weeks. The third visit was 2 weeks after the second visit when a third assessment form of subjective symptoms of VVA was completed. Two and 4 weeks after completion of the intervention, a follow-up phone call was made and assessment forms for subjective symptoms of VVA were again completed based on the answers that patients provided over the phone. The aim of follow-up 2 and 4 weeks from the end of the intervention was to evaluate the recurrence of symptoms after discontinuation of the medication. Patients also filled out a side-effect assessment form during the first, second, and third visits. In addition, at the end of the intervention, a patient satisfaction survey was performed based on a 4-point Likert scale (low satisfaction, intermediate, high, and very high).
3.4. Statistical Analysis
Data analysis was performed, using the SPSS software, version 24.0 was used for statistical data analysis. First, the descriptive statistics (mean and standard deviation) were reported for the VVA subjective symptoms scores (VVA-SSS). The paired-sample t test was used to assess the effect of treatment on VVA. Then, the marginal modeling and Generalized Estimating Equations (GEE) methodology were utilized to evaluate the longitudinal effect of treatment on mean VVA-SSS over the study period. Statistical significance was defined as P-values less than 0.05.
3.5. Ethical Approval
After approval of the investigational review board at Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1398.437) and registration of study in the Iranian Clinical Trial Center (IRCT20191210045687N1), sampling started.
4. Results
Out of 84 women, who were initially assessed for eligibility to participate in this study, 50 were included (Figure 1)
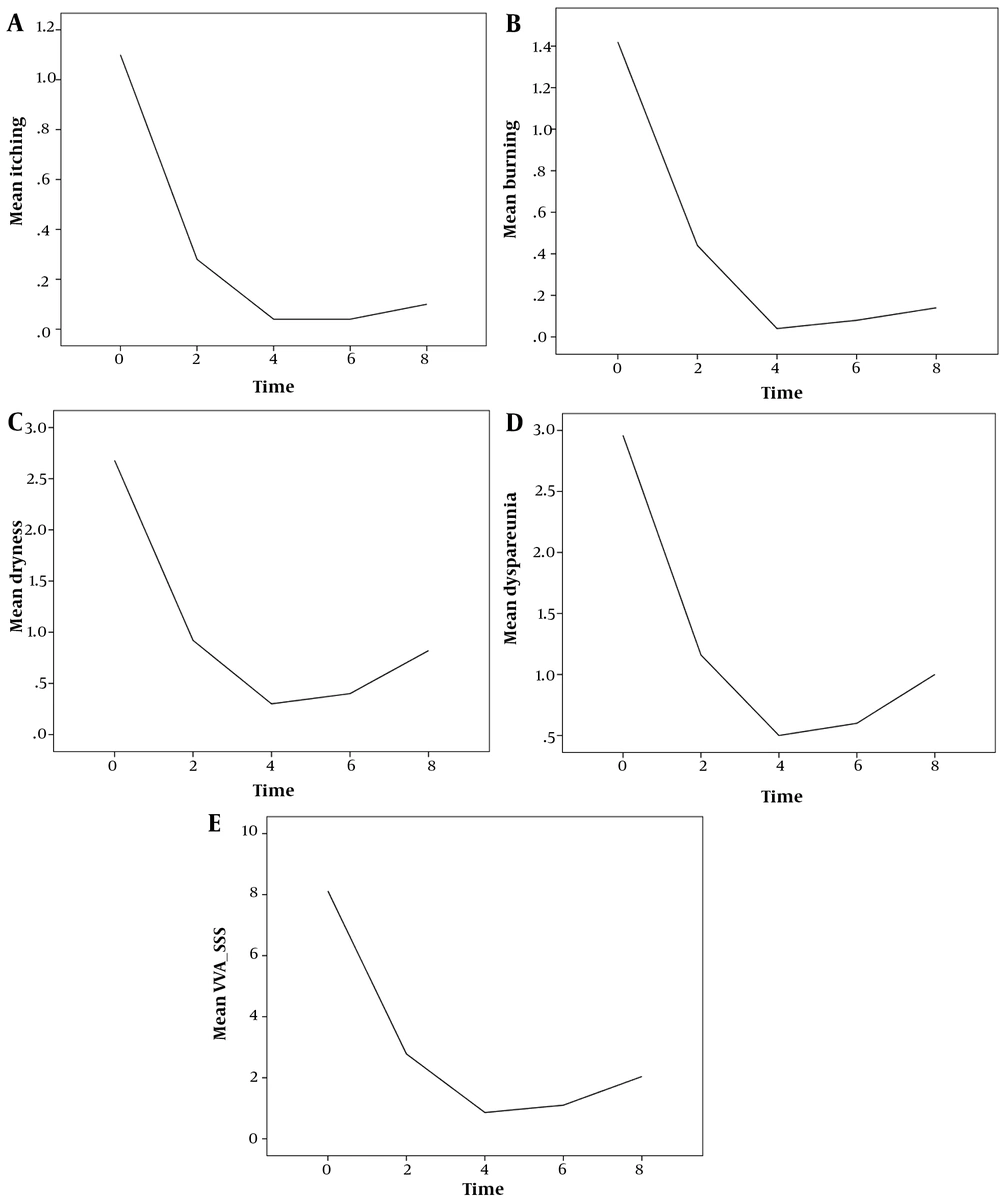
Results of the monitored indices (itching, burning, dryness, dyspareunia, and VVA- SSS) are summarized in Table 1. The mean scores for itching, burning, dryness, dyspareunia and VVA-SSS decreased for 6 weeks after beginning the intervention (until 2 weeks after completion of the intervention) and increased slightly in week 8 (Table 1).
| Indices/Time a | Minimum | Maximum | Mean ± SD |
|---|---|---|---|
| Itching score | |||
| 0 | 0.00 | 4.00 | 1.10 ± 1.16 |
| 2 | 0.00 | 2.00 | 0.28 ± 0.54 |
| 4 | 0.00 | 1.00 | 0.04 ± 0.20 |
| 6 | 0.00 | 1.00 | 0.04 ± 0.20 |
| 8 | 0.00 | 1.00 | 0.10 ± 0.30 |
| burning score | |||
| 0 | 0.00 | 3.00 | 1.42 ± 1.09 |
| 2 | 0.00 | 2.00 | 0.44 |
| 4 | 0.00 | 1.00 | 0.04 |
| 6 | 0.00 | 2.00 | 0.08 |
| 8 | 0.00 | 2.00 | 0.14 |
| dryness score | |||
| 0 | 0.00 | 4.00 | 2.68 |
| 2 | 0.00 | 3.00 | 0.92 |
| 4 | 0.00 | 2.00 | 0.30 |
| 6 | 0.00 | 2.00 | 0.40 |
| 8 | 0.00 | 3.00 | 0.82 |
| Dyspareunia score | |||
| 0 | 1.00 | 4.00 | 2.96 |
| 2 | 0.00 | 2.00 | 1.16 |
| 4 | 0.00 | 2.00 | 0.50 |
| 6 | 0.00 | 3.00 | 0.60 |
| 8 | 0.00 | 3.00 | 1.00 |
| VVA-SSS | |||
| 0 | 4.00 | 15.00 | 8.12 |
| 2 | 0.00 | 7.00 | 2.78 |
| 4 | 0.00 | 4.00 | 0.86 |
| 6 | 0.00 | 5.00 | 1.10 |
| 8 | 0.00 | 7.00 | 2.04 |
Abbreviation: VVA-SSS, vulvovaginal atrophy subjective symptom score.
a Time: 0 = baseline (before intervention), 2 = 2 weeks after the intervention beginning, 4 = 4 weeks after the intervention beginning, 6 = 2 weeks after the intervention completion, 8 = 4 weeks after the intervention completion.
The mean score of vaginal itching decreased during the first 4 weeks of intervention and later increased slightly following the discontinuation of the intervention (Figure 2A).
The mean score of vaginal burning decreased during the first 4 weeks of intervention and later increased slightly following the discontinuation of the intervention (Figure 2B).
The mean score of vaginal dryness decreased during the first 4 weeks of intervention and later increased slightly following the discontinuation of the intervention (Figure 2C).
The mean score of dyspareunia decreased during the first 4 weeks of intervention and later increased slightly following the discontinuation of the intervention (Figure 2D).
The mean score of VVA-SSS decreased during the first 4 weeks of intervention and later increased slightly following the discontinuation of the intervention (Figure 2E).
To evaluate the longitudinal effect of treatment on mean vaginal itching, vaginal burning, vaginal dryness, dyspareunia, and VVA subjective symptom scores over the study period, the marginal modeling and GEE methodology was used. The results are shown in Table 2.
| Dependent Variable/Parameter | EST | SE | P-Values |
|---|---|---|---|
| Itching | |||
| Intercept | 1.10 | 0.16 | > 0.001 |
| Baseline | Ref | --- | --- |
| Time = 2 | -0.82 | 0.12 | > 0.001 |
| Time = 4 | -1.06 | 0.15 | > 0.001 |
| Time = 6 | -1.06 | 0.15 | > 0.001 |
| Time = 8 | -1.00 | 0.14 | > 0.001 |
| Burning | |||
| Intercept | 1.42 | 0.15 | > 0.001 |
| Baseline | Ref | --- | --- |
| Time = 2 | -0.98 | 0.11 | > 0.001 |
| Time = 4 | -1.38 | 0.15 | > 0.001 |
| Time = 6 | -1.34 | 0.15 | > 0.001 |
| Time = 8 | -1.28 | 0.14 | > 0.001 |
| Dryness | |||
| Intercept | 2.68 | 0.13 | > 0.001 |
| Baseline | Ref | --- | --- |
| Time = 2 | -1.76 | 0.11 | > 0.001 |
| Time = 4 | -2.38 | 0.12 | > 0.001 |
| Time = 6 | -2.28 | 0.11 | > 0.001 |
| Time = 8 | -1.86 | 0.12 | > 0.001 |
| Dyspareunia | |||
| Intercept | 2.96 | 0.12 | > 0.001 |
| Baseline | Ref | --- | --- |
| Time = 2 | -1.80 | 0.10 | > 0.001 |
| Time = 4 | -2.46 | 0.12 | > 0.001 |
| Time = 6 | -2.36 | 0.13 | > 0.001 |
| Time = 8 | -1.96 | 0.14 | > 0.001 |
| VVA Subjective symptoms | |||
| Intercept | 8.12 | 0.38 | > 0.001 |
| Baseline | Ref | --- | --- |
| Time = 2 | -5.34 | 0.25 | > 0.001 |
| Time = 4 | -7.26 | 0.32 | > 0.001 |
| Time = 6 | -7.02 | 0.31 | > 0.001 |
| Time = 8 | -6.08 | 0.32 | > 0.001 |
Abbreviations: EST, estimate; SE, standard error; Ref, reference category.
Based on the results listed in Table 2, the maximum decline in mean scores of vaginal itching was observed 4 weeks after the beginning of therapy and 2 weeks after completion of the intervention (Approximately 1.06 decline in score compared to baseline), and the maximum decline in mean scores of vaginal burning, vaginal dryness, dyspareunia, and VVA subjective symptoms was observed 4 weeks after the beginning of therapy (Approximately 1.38, 2.38, 2.46, and 7.26 decline in score compared to baseline). The decline from baseline was statistically significant at all time points throughout the study (P < 0.001).
The patient satisfaction at the end of the intervention was very high in 84% (42 of 50 patients), high in 12% (6 patients), and moderate in 4% (2 patients). None of the participants reported any medication side effects.
5. Discussion
This study aimed at investigating the effect of a new vaginal cream (A Persian medicine product) on subjective symptoms of VVA in BCS. The results showed that the use of this drug for 4 weeks decreased vaginal itching, vaginal burning, vaginal dryness, and dyspareunia. The difference in the severity of the subjective symptoms before treatment and 4 weeks after completion of treatment was statistically significant.
Low circulating estrogen level leads to VVA with subjective symptoms including vaginal dryness, itching, burning, and dyspareunia. Systematic therapies including chemotherapy and endocrine therapy for the management of breast cancer can lead to persistent estrogen suppression, which may cause atrophic vaginitis in a large proportion of a variety of BCS. Vaginal estrogen therapies are the most effective treatments for VVA symptoms, but their use in BCS has been controversial. The current treatment options available for VVA are non-hormonal treatments, local estrogen therapy, vaginal testosterone, and fractional CO2 laser/erbium laser (18).
Based on a randomized controlled trial (RCT) on 40 menopausal women with vaginal atrophy, Ziagham et al. (27) reported that administration of either 5 mg hyaluronic acid sodium salt or 1 mg vitamin E for 8 week in the form of vaginal suppositories improved the signs and symptoms of VVA significantly in both groups and improvements were greater in the hyaluronic acid group. Jokar et al. (28) reported an RCT on 56 menopausal women studying the effects of conjugated Estrogen cream 0.625 mg (hormonal) compared to hyaluronic acid cream (non-hormonal) for the treatment of atrophy. They showed that hyaluronic acid may be an appropriate alternative option for conjugated estrogen in women with genital system atrophy and those with medical contraindications or side effects of hormonal drugs. Juraskova et al. (29) showed that dyspareunia, sexual function, and quality of life after breast cancer significantly improved using olive oil (as a lubricant), vaginal moisturizer, and pelvic floor muscle relaxation. Chiu et al. (30) reported that according to a meta-analysis of 7 RCTs, acupuncture has small impact on severity of menopause-related symptoms in BCS immediately following 5 - 15 sessions of acupuncture and that the effects on the severity of menopause-related symptoms could persist at the time of 1 - 3 month follow-up. This meta-analysis concluded that acupuncture could be considered a useful addition to the conventional therapies for BCS experiences menopause-related symptoms; however, whether acupuncture has specific treatment effects more than needling or placebo effects, needs to be further evaluated.
Asgharpoor and Rahmany (31), in their systematic review of 12 clinical trials out of 747 articles, noted that 7 medicinal plants containing phytoestrogens (chamomile, Pueraria mirifica, licorice, flaxseed, black cohosh, fennel, and red clover) Herbal products can have beneficial effects on reducing the symptoms of vaginal atrophy in postmenopausal women and can be considered a treatment. The overall results obtained from the review of existing studies indicate that these plants have Phytoestrogenic properties and act like the natural body's estrogens in a less potent form. Knight et al. (32) showed in a systematic review that 6 studies out of 52 studies investigated the benefits of laser therapy in improving vaginal symptoms of VVA in women following breast cancer treatment. This review expressed that several small-scale studies suggested an improvement in vaginal health in women, who had had breast cancer, both objectively and subjectively, and there were no large-scale randomized studies that discussed the acceptability of the intervention and, therefore, highlighted a need for large prospective randomized controlled trials in order to study the benefits of vaginal laser therapy in cases of vaginal atrophy and to better understand whether such treatment can ameliorate symptoms and improve quality of life in postmenopausal patients, particularly after breast cancer treatment.
The medicinal product used in our study is made of chicken tallow and beeswax. Chicken tallow contains a combination of oleic acid, linoleic acid, and palmitic acid and has a large amount of water (33). Beeswax is a rich source of vitamin A, 100 grams of beeswax contains 4096 IU of vitamin A, and vitamin A in wax is easily absorbed through the skin. Beeswax has an anti-inflammatory characteristic with healing properties for wounds and burns, as well as antibiotic effects, such that Hippocrates used it to heal wounds (34, 35).
Previous studies have suggested different mechanisms, by which non-hormonal vaginal lubricants and moisturizers can relieve VVA symptoms. Hyaluronic acid or vitamin E can improve tissue hydration levels and have anti-inflammatory activity (27, 28). Olive oil consists of mainly oleic acid and small amounts of other fatty acids such as linoleic acid and palmitic acid; this is similar to the composition of chicken tallow (29). Vitamin A is a fat-soluble vitamin that stimulates epidermal turnover, increases the rate of re-epithelialization, and restores epithelial structure (36). Therefore, the mechanism of action of our proposed drug may be by moisturizing, relieving inflammation, and modifying the vaginal epithelial lining. The effectiveness of our drug in providing relief and reducing symptoms of vaginal atrophy is similar to the study done by Ziagham et al. (27).
This is the first study on a medicinal product with compounds of animal origin to treat subjective symptoms of VVA. The proposed medicine is relatively inexpensive, and no side effects were reported.
5.1. Conclusions
The study was conducted in the form of a before-after clinical trial that was performed on 50 postmenopausal BCS above 18 years of age. The results showed that our novel vaginal cream, rooted in Persian Traditional Medicine, may be a safe and effective therapy to relieve subjective symptoms of VVA in BCS.
5.2. Limitation and Recommendation
The limitations of our study include the lack of a control group (placebo or another drug group) because each placebo in the form of a cream has at least some effect on this disease, and some drugs have estrogenic effects; so, their usage in BCS is controversial and some of them are expensive, imported, and not easily available. Future work may include controlled-trial with the longer follow-up of patients and assessing various doses and durations of the cream used to compare effectiveness.