1. Background
According to GLOBOCAN 2020, cervical cancer is ranked as the 4th most prevalent cancer (3.1%) and the 4th major cause of cancer death (3.4%) in women (1). Generally, persistent infections with human papillomaviruses (HPVs), especially high-risk types such as HPV16 and HPV18, are responsible for more than 90% of cervical cancer cases (2). Pelvic surgery and chemotherapy are the common methods in the treatment of early and advanced cervical cancer (3). However, chemotherapeutic agents have many side effects, including neurotoxicity, reproductive toxicity, and cardiotoxicity, limiting their function and application for cancer therapy (4-6). Therefore, identifying novel anti-cancer drugs with minimal side effects and efficient therapeutic effects for cervical cancer treatment is urgent.
Histone deacetylases (HDACs) could modulate gene expression via removing acetyl groups from core histones and non-histone proteins, resulting in chromatin condensation and suppressed gene expression (7). In many cancers, changes in the function of HDAC enzymes lead to alteration and regulation in gene expression and protein function. HDAC inhibitors, such as scriptaid, LBH589, quisinostat, and sodium butyrate, could reactivate suppressed genes and subsequently sensitize tumor cells to chemotherapy agents, inhibit cell proliferation and metastasis, and induce apoptosis (8). Valproic acid (VPA), an HDAC inhibitor, is a branched short-chain fatty acid used to treat various types of epilepsy (9). In recent years, the anti-tumor effects of VPA on numerous types of cancers, such as colon, pancreatic, thyroid, breast, hepatocellular, and cervical cancers, have attracted more attention (10-15). For example, VPA suppressed tumor growth of prostate cancer via inducing autophagy and inhibiting the Akt/mTOR pathway (16). Moreover, VPA can exert its anti-proliferative effects by inducing apoptosis and cell cycle arrest as well as inhibiting metastasis and angiogenesis (17-20). The anti-cancer effects of VPA in combination with other therapeutic agents also have been demonstrated. For instance, VPA potentiated the anti-tumor activities of a cyclin-dependent kinase blocker, P276-00, by upregulating p21, p27, and p53, and downregulating cyclin D1 and Bcl2 (21). Although some studies showed that VPA inhibits tumor growth via promoting caspase-dependent apoptosis, enhancing chemosensitivity, and inhibiting angiogenesis (19-21), there is insufficient data on the VPA effect on cervical cancer cells.
2. Objectives
This study aimed at examining the role of VPA on cultured HeLa cells, a typical cervical cancer cell line, and investigating its underlying molecular mechanism, particularly in cell apoptosis and cell cycle.
3. Methods
3.1. Valproic Acid Preparation
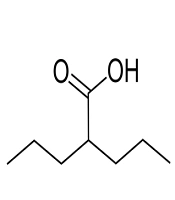
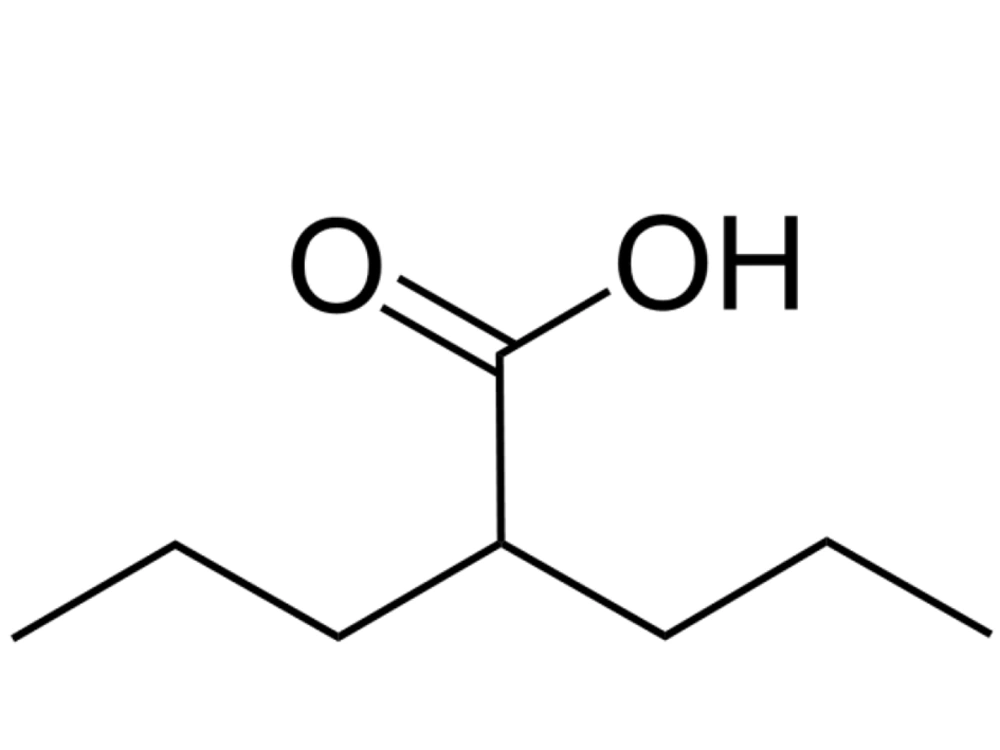
Valproic acid (Damavand Darou, Iran) was dissolved in sterile water, sterilized through a 0.22-μm filter, and stored at -20°C. The stock of VPA was diluted to the desired concentration (6.25, 12.5, 25, 50, and 100 mM). The chemical structure of VPA is presented in Figure 1.
3.2. Cell Culture
HPV-positive cell line, HeLa cells, was obtained from the Iranian cell bank, Pasteur Institute of Iran. HeLa cells were cultured in Dulbecco’s modified eagle medium (DMEM, Gibco, USA) containing 10% fetal bovine serum (FBS, Gibco) and antibiotics, including 1% penicillin/streptomycin at 37°C with 5% CO2. HeLa cells in the logarithmic growth phase were selected for further experiments.
3.3. Cell Viability Assay
The 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay was used to evaluate the effect of VPA on the viability of HeLa cells. Briefly, 2 × 103 HeLa cells (per well) were seeded in 96-well plates and incubated for 24 h. After incubation, the cells were exposed to different concentrations of VPA (range: 6.25 - 100 mM) in a 1% FBS-containing medium for 24, 48, and 72 h. The untreated cells were used as the control group. Then, the medium was replaced by 100 μL of MTT (0.5 mg/mL) in a 1% FBS-containing medium, and cells were incubated at 37°C in the CO2 incubator for 3 h. After removing supernatants, the formed formazan was solubilized by adding 100 μL of dimethyl sulfoxide (DMSO, Sigma-Aldric, USA). Absorbance at a wavelength of 570 nm was determined, using a microplate reader (ELx 800, BioTek, USA). The experiment was repeated 3 times.
3.4. Apoptosis Assay
To assess the effects of VPA on apoptosis of HeLa cells, 5 × 104 cells (per well) were cultured in a 6-well plate and incubated at 37°C overnight. HeLa cells were treated with the indicated concentrations of VPA for 48 h. Then, the cells were trypsinized, washed twice with cold PBS, and resuspended with FITC/annexin V and Propidium Iodide (PI) (BD Biosciences, USA) for 30 min at 25°C in the dark and, then, analyzed by a flow cytometer analyzer (BD FACS Calibur, BD Biosciences, USA). The experiment was repeated 3 times.
3.5. Cell Cycle Assay
For cell cycle assay, HeLa cells were trypsinized, washed once with cool PBS, and fixed in pre-cooled 75% ethanol overnight. Subsequently, the cells were washed with cool PBS and stained with 0.5 mL PI/RNase Staining Buffer for 15 min in darkness. The results were analyzed, using flow cytometry (BD FACS Calibur, BD Biosciences, USA). The experiment was repeated 3 times.
3.6. Real-Time PCR
HeLa cells at 1 × 106 cells/well were plated on a 6-well plate and treated with VPA at 0, 12.5, 25, and 50 mM for 48 h. Following treatment, the cells were harvested and washed with 500 μL of PBS. Total RNA was extracted, using RNX-Plus solution (SinaClon, Tehran, Iran) according to the manufacturer’s instructions and quantified, using a nanodrop (Thermo Fisher Scientific, CA). Reverse transcription was performed, using the complementary DNA (cDNA) synthesis kit (Yekta‐Tajhiz, Iran). The expression of Bax, Bcl-2, p53, and p21 genes was determined by using SYBR Green RTPCR reagents (Ampliqon, Denmark) and specific primers (Table 1). The general conditions of real‐time PCR were 95°C for 5 min, 95°C for 15 sec, 59°C to 64°C for 45 sec, and 72°C for 30 sec; 40 cycles. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an inner reference gene to normalize the apoptotic genes’ expression. Cycle threshold (Ct) values were calculated by normalizing the mRNA level of the target gene to GAPDH mRNA and the relative gene expression level was represented as 2-ΔΔCt. The experiment was repeated 3 times.
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Bcl-2 | 5’-TGTGGCCTTCTTTGAGTTCG-3’ | 5’-TCACTTGTGGCCCAGATAGG-3’ |
| Bax | 5’-GCGTCCACCAAGAAGCTGAG-3’ | 5’-ACCACCCTGGTCTTGGATCC-3’ |
| p53 | 5’-ACTAAGCGAGCACTGCCCAA-3’ | 5’-ATGGCGGGAGGTAGACTGAC-3’ |
| p21 | 5’-TATGGGGCTGGGAGTAGTTG-3’ | 5’-AGCCGAGAGAAAACAGTCCA-3’ |
| GAPDH | 5’-GAAGGTGAAGGTCGGAGTCA-3’ | 5’-GACAAGCTTCCCGTTCTCAG-3’ |
3.7. Statistical Analysis
The data were analyzed, using GraphPad Prism 8 (GraphPad Software Inc.). The experiments were performed in triplicate and expressed as the means ± standard deviation (SD). Statistical analysis was performed, using one-way and two-way ANOVA tests followed by Tukey’s multiple comparison test. The differences were considered significant for P < 0.05.
4. Results
4.1. Valproic Acid Inhibits the Proliferation of HeLa Cells
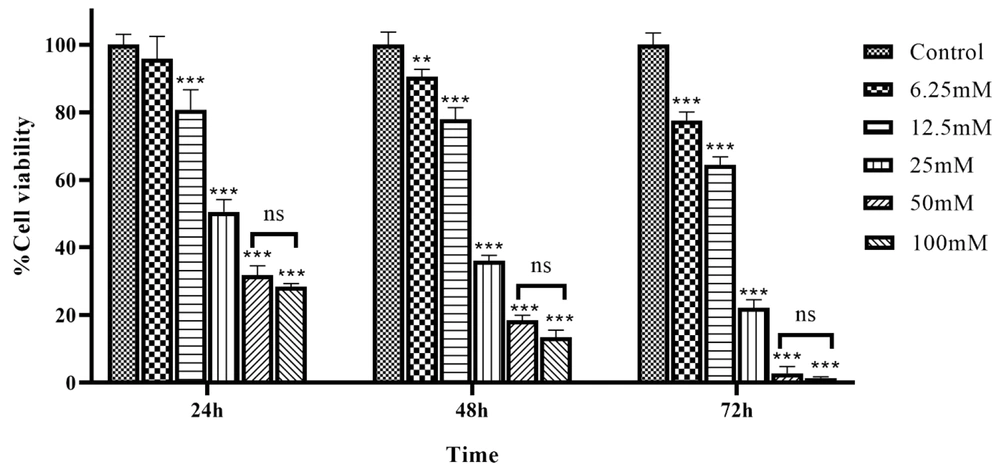
The cytotoxicity effect of VPA at concentrations ranging from 6.25 to 100 mM for 24, 48, and 72 h on HeLa cells was evaluated by the MTT assay. The results showed that VPA inhibited the proliferation of the HeLa cell line in a dose-dependent and time-dependent manner (Figure 2). The IC50 values of VPA on HeLa cells at 24, 48, and 72 h were 32.06, 21.29, and 14.51 mM, respectively.
Anti-proliferative activity of valproic acid on HeLa cell line. Cells were treated with 0 - 100 mM VPA and incubated for 24, 48, and 72 h. Data are presented as the mean ± standard deviation of 3 independent experiments. ** P < 0.01 compared to control group, *** P < 0.001 compared to control group, ns no significant.
4.2. Valproic Acid Induces Apoptotic Cell Death of HeLa Cells
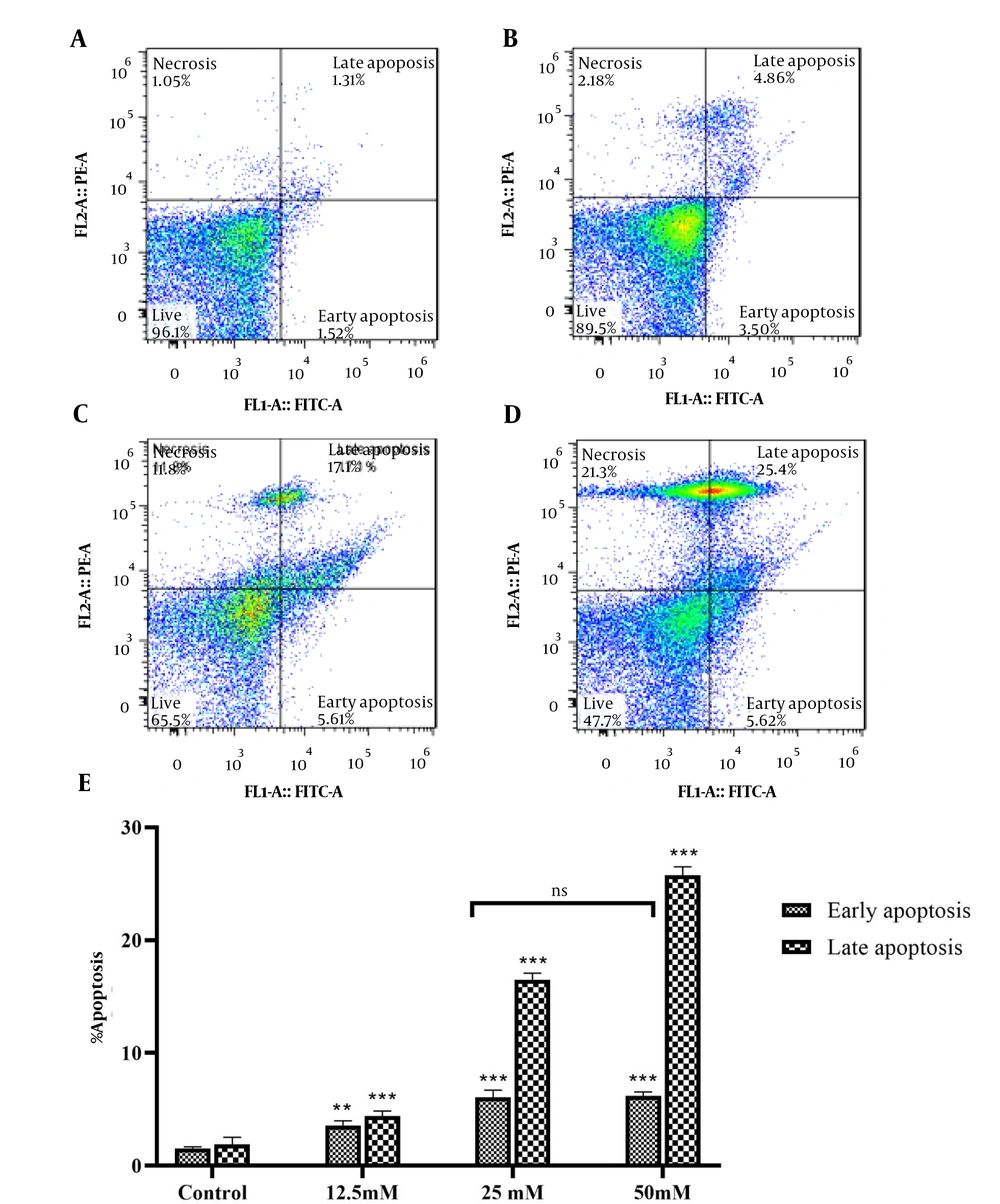
To investigate apoptosis induction by VPA, flow cytometry was carried out after different concentrations of VPA were added into the culture medium of HeLa cells. After incubation with 12.5, 25, and 50 mM of VPA for 48 h, the percentage of late apoptosis (Annexin V+/PI+) cells were 4.86%, 17.10%, 25.40%, and, respectively, as compared to 1.31% in the control group. Furthermore, early apoptosis (Annexin V+/PI-) cells were 3.5%, 5.61%, and 5.80%, respectively, as compared to 1.52% in the control group (Figure 3).
Analysis of the cell apoptosis with annexin-V/PI staining, using flow cytometry. A, the early and late apoptosis of control group; and B, the groups treated with VPA at 12.5; C, 25; and D, 50 mM concentration for 48 h. E, data are mean ± SD of 3 treatments in each group. ** P < 0.01 compared to control group; *** P <0 .001 compared to control group; ns, no significant.
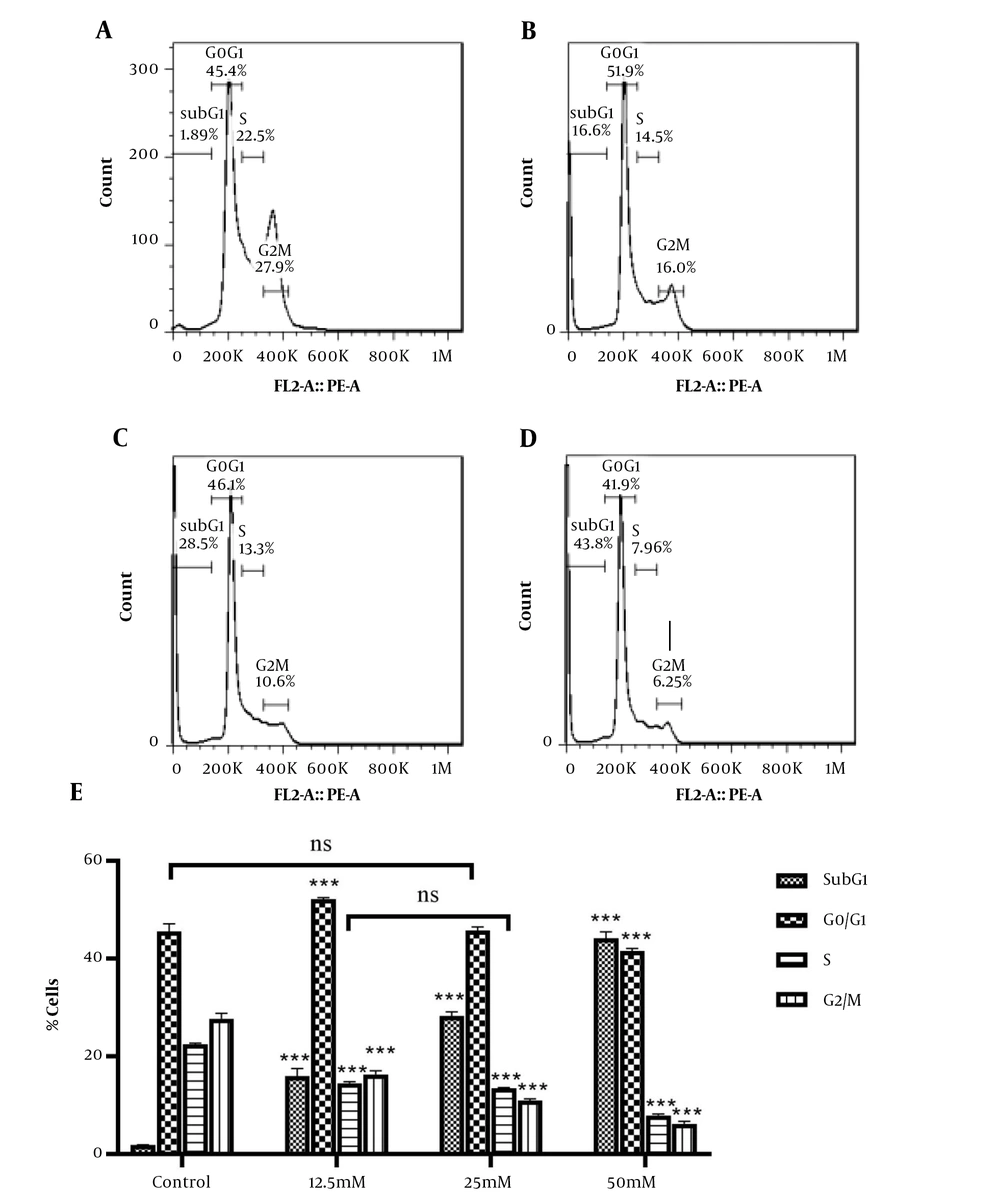
4.3. Valproic Acid Induces Cell Cycle Arrest of HeLa cells
To assess the impact of VPA on cell cycle progression in HeLa cells, the DNA content was measured, using flow cytometry. Figure 4 shows that after 48 h of treatment with 12.5, 25, and 50 mM of VPA, there was a marked increase in the sub-G1 population in HeLa cells, confirming apoptosis induction after 48 h. In addition, treatment with VPA led to a significant reduction in G2-M phase populations.
Cell cycle analysis of HeLa cells under VPA exposure, using flow cytometry. A, the population of cells in each phase of the cell cycle in the control group; and B, the groups treated with VPA at 12.5; C, 25; and D, 50 mM concentration for 48 h. E, data are mean ± SD of 3 treatments in each group. *** P < 0.001 compared to the control group; ns, no significant.
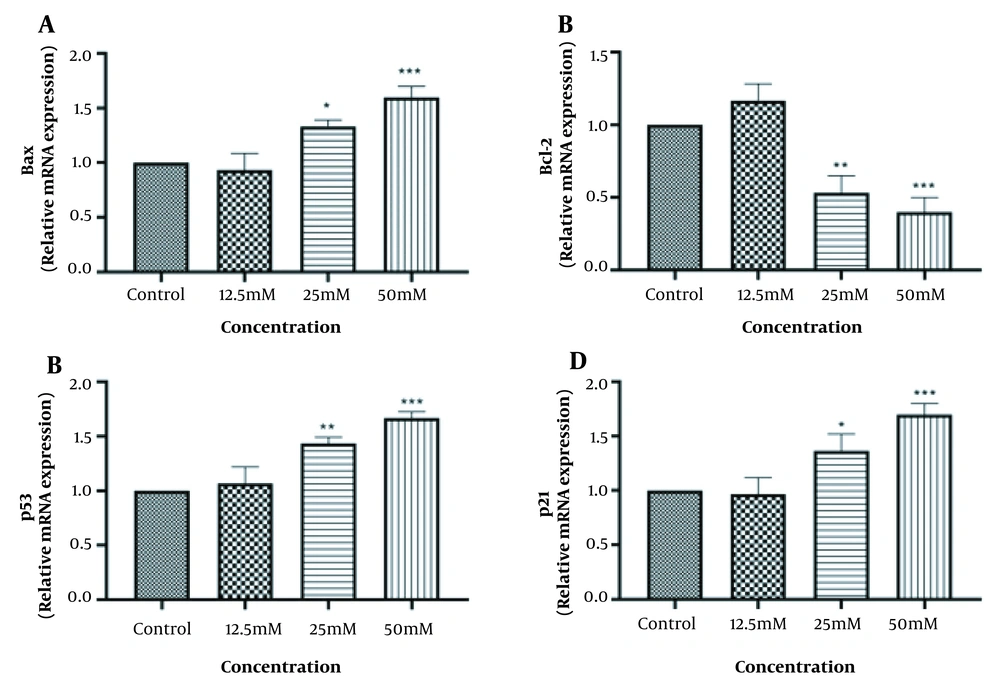
4.4. Valproic Acid Induces the Intrinsic Pathway of Apoptosis in HeLa cells
To determine the molecular mechanism of VPA-induced apoptosis in HeLa cells, the mRNA levels of several apoptosis-related genes, including Bax, Bcl-2, p53, and p21 were examined. As a result, VPA increased the expression of Bax (a pro-apoptotic marker) and decreased the expression of Bcl-2 (an anti-apoptotic marker) in a dose-dependent manner (Figure 5A and B). Since p53 protein is an upstream effector of p21 protein, as well as its regulatory effect on the Bax upregulation and Bcl-2 downregulation, the expression of p53 and p21 were tested. The overexpression of the mRNA levels of p53 and p21, as shown in Figure 5C and D, revealed that VPA induced the upstream signaling of the intrinsic pathway of apoptosis.
Effect of VPA on markers of apoptosis-related genes in HeLa cells after 48h. A, the expression level of Bax; B, the expression level of Bcl-2; C, the expression level of p53; D, the expression level of p21; E, data are mean ± SD of 3 treatments in each group. * P < 0.05 compared to the control group; ** P < 0.01 compared to the control group; *** P < 0.001 compared to the control group.
5. Discussion
Valproic acid as a histone deacetylase inhibitor exerts anti-tumor effects on various cancer cells by inhibiting cancer cell migration and metastasis, modulating inflammation and immune responses, and inducing apoptosis and cell cycle arrest (22, 23). The present study aimed at determining the cytotoxic activity of VPA against the human cervical cancer cell line, HeLa, and its possible mechanisms of action. We found that VPA inhibits the proliferation of HeLa cells by inducing cell cycle arrest and apoptosis. Further analysis revealed that VPA exerts its pro-apoptotic property by enhancing p53 and p21 expression and inducing the intrinsic pathway of apoptosis in HeLa cells.
We demonstrated that VPA significantly inhibited the proliferation of HeLa cells in a concentration- and time-dependent manner. This finding is consistent with other previous studies indicating the anti-proliferative function of VPA in other tumor cell lines, including glioma (24), multiple myeloma (25), prostate (26), bladder (27), gastric (28), and urothelial (29) cancers. After VPA exposure, decreased cell proliferation in HeLa cells has been previously reported (15, 30). Therefore, VPA could be a potential choice for the development of an anti-cancer agent for targeting cervical cancer.
Apoptosis initiates through 2 pathways: (1) Extrinsic (death receptor) which begins with the binding of death ligands to death receptors and activates caspase-8, and (2) intrinsic (mitochondrial) that occurs within the cells as a result of increased mitochondrial permeability and, then, the release of pro-apoptotic molecules into the cytoplasm, leading to the activation of caspase-3 and caspase-9 (31). We found that VPA remarkably induced apoptosis and cell cycle arrest in HeLa cells. We showed that the intrinsic pathway is involved in VPA-induced apoptosis. Valproic acid treatment of HeLa cells also increased the number of sub-G1 cells. In agreement with our results, Catalano et al. found that VPA treatment suppressed the growth of thyroid cancer cell lines by inducing apoptosis and cell cycle arrest (32). They showed that VPA selectively activates the intrinsic pathway of apoptosis by increasing caspase-9 activity without a significant increase in the activity of caspase-8. In contrast to our study, they indicated that VPA could arrest the cell cycle at the G1 phase (32). In another study, Wu and Guo concluded that cell cycle arrest at the G0/G1 phase and a decrease in the percentage of cells at S and G2/M phases after 16 h treatment with VPA (33).
It has been shown that p53, as a tumor suppressor, plays a vital role in controlling various cellular processes, including cell senescence, cell cycle, and cell apoptosis. During the apoptosis process, it mediates the expression of proteins that are involved in the release of mitochondrial cytochrome c, such as Puma, Noxa, AIP1, APAF1, and Bax (34). We also found that VPA treatment upregulated the expression of Bax, suggesting this may be owing to the transcriptional activation function of the p53 protein. As our results showed, p53 was upregulated upon VPA treatment. In addition, the exposure of HeLa cells with VPA led to the upregulation of p21, a cyclin-dependent kinase 2 (CDK2) inhibitor that is transcriptionally activated via p53. Some studies support the role of VPA treatment in the upregulation of p53 and p21. For instance, Sanaei and Kavoosi demonstrated that VPA could downregulate HDAC 1, 2, and 3, and anti-apoptotic genes, including Bcl-2, Bcl-xL, and Mcl-1, and upregulate pro-apoptotic genes, including Bax, Bak, Bim, p53, and p21 in HepG2 cell line, suggesting that VPA induces intrinsic pathway of apoptosis (35). Mechanistically, VPA increases the pro-apoptotic activity of p53 at the mitochondrial membrane by stabilizing its acetyl modification at lysine 120 (36). Following the acetylation and activation, p53 translocates into the nucleus and regulates the expression of pro-and anti-apoptotic genes, including Puma, Survivin, Bcl-2, and Bax (37). In contrast to our study, evidence shows that the effects of VPA on cell cycle arrest and apoptosis do not associate with the upregulation of p53 (38). Sami et al. found that HeLa cell treatment with VPA could induce the expression of p21 with no change in p53 expression (15). Similarly, the treatment of HeLa cells with 4 mM for 72 h could remarkably increase the levels of p21 (21-fold), but slightly reduce the p53 expression (-1.85-fold) (39). These controversial effects may be the results of using different cell types and different concentrations of VPA. For instance, Das et al. showed that different glioblastoma cell lines were arrested at different cell cycle phases in response to VPA treatment (40). Also, other HDAC inhibitors could induce apoptosis via enhancing p53 acetylation. De et al. found that MHY2256 could arrest the cell cycle and induce apoptosis through the acetylation of p53 in endometrial cancer (41). In another study, Bao et al. showed that the anti-metastasis and pro-apoptosis activities of quisinostat are mediated by activating the p53 signaling pathway in lung cancer (42). Quisinostat induced acetylation of p53 at K382/K373 sites, which led to upregulation of p21 and cell arrest at the G1 phase (42).
In this study, we tried to evaluate the anti-cancer activities of VPA on the HeLa cell line. The results of the study could provide more reliable information if other cervical cancer cell lines were also utilized in the experiments. Moreover, including an in vivo tumor model and investigating the tumor volume following the administration of VPA could give us reliable results. According to the obtained in vitro results, combining VPA with other therapeutic agents and assessing their optimal conditions could be considered for further studies.
5.1. Conclusions
In conclusion, our study demonstrated that VPA is a potential anti-cancer agent owing to its ability to decrease the proliferation of HeLa cervical cancer cells and induction of cell cycle arrest. In addition, VPA promotes apoptosis of HeLa cells through induction of the intrinsic pathway of apoptosis in a p53-dependent manner.