1. Background
Autologous hematopoietic stem cell transplantation (autoHSCT) is an efficient way to treat multiple myeloma and lymphoma (1). Two primary criteria for successful transplantation are stable and timely engraftment (2). Based on previous reports, CD34+ cells’ dose could highly influence engraftment (3-5). Transplantations were primarily performed using bone marrow-derived stem cells, whereas peripheral blood (PB) is currently the main source of stem cells among all transplants (5). For this purpose, stem cell mobilizers are administered to accelerate stem cell production in the bone marrow and their mobilization to peripheral blood. Granulocyte-colony stimulating factor (G-CSF) is found to be the most common substance to stimulate the BM. However, an adequate stem cell dose cannot be obtained in some patients due to various factors, including previous chemotherapy, bone marrow involvement, and pre-apheresis thrombocytopenia (6). Limitations in collecting the optimum stem cell dose have elucidated the importance of novel agents for poor mobilizer patients (7). The critical role of the CXCR4/SDF1 axis in stem cell homing could never be overstated. Blocking the CXCR4/SDF1 axis could potentially facilitate stem cell egress from the BM to blood circulation. Plerixafor, a specific inhibitor of CXCR4, is on top of the growing list of novel mobilizers (8). The European Medicines Agency has recently approved this drug for hematopoietic stem cells (HSCs) mobilization combined with G-CSF. According to our previous case report, plerixafor administration positively affected mobilization in a refractory multiple myeloma patient with extensive bone marrow involvement (9).
The most suitable time for plerixafor administration remains to be determined. It has been revealed that it takes 11 hours for the drug to reach its highest concentration in healthy donors’ blood. The more accurate information we get about the ideal time for plerixafor administration, the higher number of CD34+ cells we can collect (10).
2. Objectives
In this study, we aimed at evaluating the level of peripheral blood CD34+ cells at plerixafor administration time and every three hours to identify the peak time of circulating CD34+ cells.
3. Methods
3.1. Patients
This single-center prospective study was designed to determine when CD34+ cells number rose to peak level in peripheral blood after the use of plerixafor for stem cell mobilization. A total number of 25 hematopoietic stem cell transplantation candidates with plerixafor-mobilized CD34+, based on the discretion of the physician and treatment strategies in our center, were enrolled in this study. Patients who received melatonin for mobilization (2 patients) and patients who did not show an increase in the level of CD34+ cells after plerixafor administration (2 patients) were excluded from the study. Before admission, the status of all patients was confirmed based on our previous report (11).
3.2. HSCs Collection and Indication for Plerixafor
G-CSF (filgrastim) was injected subcutaneously at a dosage of 5 - 10 μg/kg/day for 4 - 5 consecutive days. Based on Flow cytometry enumeration on the fourth day, plerixafor administration is recommended for patients whose CD34+ dose was absolutely low (< 5/uL), relatively low (5 - 10/uL), and borderline (10 - 20/uL).
Plerixafor (Neofar, biosimilar Nanoalvand Company) was subcutaneously administrated at a dose of 0.24 mg/kg on day 5 at 7:00 AM. 4 mL of peripheral blood was collected every three hours from 6:00 AM until 7:00 PM to measure CD34+ cell dose and define stem cell peak. CD34+ cells harvesting and enumeration were performed based on our previous report (12).
3.3. Statistical Analysis
The categorical variables with frequencies and percentages, the normally distributed continuous variables with mean ± SD, and the non-parametric variables with median and simple range have been reported. Outcome variables were PB CD34+ cells and CD34+ in apheresis product. PB CD34+ cells were measured before plerixafor injection and 3, 6, 9, and 12 hours after plerixafor injection. The normal distribution of outcomes was assessed by a normal test. Analysis of the variance repeated measures was used to examine within-subject effects. Bonferroni test was used for pairwise comparisons among means. The significance level was set at 0.05. Simple and multiple linear regression was performed for CD34+ cell dose in apheresis product. The significant level for simple and multiple analyses was assigned to 0.20 and 0.05, respectively. The calculations were carried out using SAS (version 9.4; SAS Institute Inc., Cary, NC, USA).
4. Results
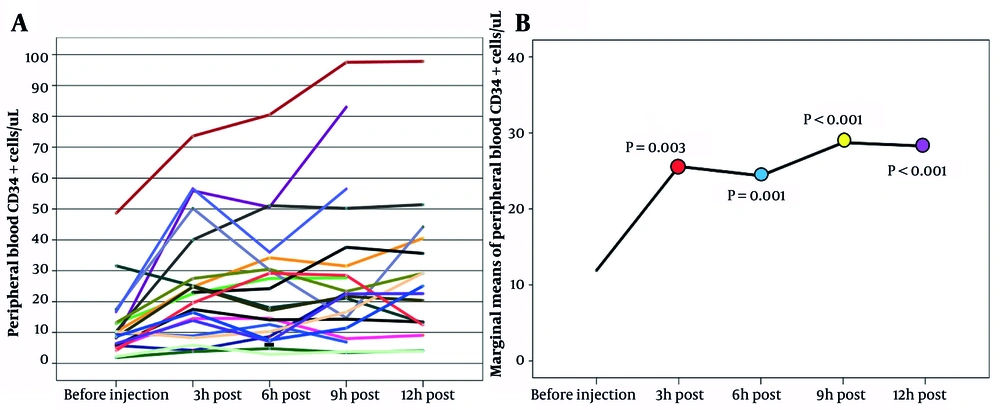
This study included 21 patients diagnosed with Hodgkin's and non-Hodgkin's lymphoma, multiple myeloma, and primitive neuroectodermal tumors. The clinical characteristics of patients are shown in Table 1. Eleven patients were treated with lenalidomide. The mean number of pre-plerixafor PB CD34+ was 11.23 ± 10.28/uL. Pre-plerixafor PB CD34+ cell dose in 11 out of 21 patients was < 10/uL. CD34+ cells mobilization in one patient reached a peak at 6 hr post-plerixafor injection but was a poor mobilizer (CD34 < 10/uL) (Figure 1A). CD34+ cell content in the apheresis product of this patient was < 2 × 106 dose/kg. The median of WBC and platelet engraftment was +11 and +10, respectively.
| Characteristics | Values a |
|---|---|
| Age (y) | 47.65 ± 13.9 |
| Missing | 1 (4.8) |
| Gender | |
| Male | 13 (61.9) |
| Female | 8 (38.1) |
| Missing | 0 (0) |
| Diagnosed disease | |
| Hodgkin | 2 (9.5) |
| Non-hodgkin | 6 (28.6) |
| Multiple myeloma | 12 (57.1) |
| PNET | 1 (4.8) |
| Missing | 0 (0) |
| Addicting | |
| Yes | 2 (9.5) |
| No | 14 (66.7) |
| Missing | 5 (23.8) |
| Blood group | |
| A | 4 (19) |
| B | 3 (14.3) |
| AB | 1 (4.3) |
| O | 11 (52.4) |
| Missing | 2 (9.5) |
| Lenalidomide | |
| Yes | 8 (38.1) |
| No | 12 (57.1) |
| Missing | 1 (4.8) |
| Mobilization failure history | |
| Yes | 0 (0) |
| No | 3 (14.3) |
| Missing | 18 (85.7) |
| Type of chemotherapy during HSCT | |
| CEAM | 7 (33.3) |
| Melphalan | 13 (61.9) |
| Missing | 1 (4.8) |
| Peripheral blood CD34+/uL | |
| Prior plerixafor | 11.89 ± 10.86 |
| Missing | 1 (4.8) |
| 3 h post plerixafor | 25.58 ± 19.18 |
| Missing | 0 (0) |
| 6 h post plerixafor | 24.36 ± 18.96 |
| Missing | 0 (0) |
| 9 h post plerixafor | 28.72 ± 24.72 |
| Missing | 0 (0) |
| 12 h post plerixafor | 28.25 ± 23.35 |
| Missing | 5 (23.8) |
| Apheresis product CD34+/kg | 5.96 ± 3.61 |
| Missing | 0 (0) |
| Platelet | |
| Admit day | 231062.5 ± 81874.26 |
| Missing | 5 (23.8) |
| HSCT day | 96750 ± 43858.10 |
| Missing | 5 (23.8) |
| Prior GCSF | 211125 ± 68041.041 |
| Missing | 5 (23.8) |
| Prior plerixafor | 131894.73 ± 81704 |
| Missing | 2 (9.5) |
| WBC | |
| Admit day | 5205.55 ± 1479.45 |
| Missing | 4 (19) |
| HSCT day | 18205.55 ± 12922.09 |
| Missing | 4 (19) |
| Prior GCSF | 7277.77 ± 3855.35 |
| Missing | 4 (19) |
| Prior plerixafor | 29156.52. ± 13544.90 |
| Missing | 1 (4.8) |
a Values are expressed as mean ± SD or frequency (%).
Trend of PB CD34+ cells/uL following plerixafor injection, A, trajectory plot; B, marginal mean plot. The P values of the statistical comparison of marginal means of peripheral blood CD34+ cells/uL between before injection of plerixafor and after 3 hours (red), 6 hours (blue), 9 hours (yellow) and 12 hours (purple) are mentioned in the graph.
Means of the pre-plerixafor and 3 - 12 hr post-plerixafor PB CD34+ are compared in Figure 1B. The mean of PB CD34+ cells remarkably increased at 3 hr post-plerixafor. Three hours after plerixafor injection, the mean of PB CD34+ cells slightly decreased. Finally, PB CD34+ cells raised to a peak at 9 hours post-plerixafor administration. Overall, according to repeated measurement analysis, the time trend was significant, and plerixafor injection was effective for PB CD34+ cells (P-value < 0.0001, ω2 = 0.083). The impact rate of plerixafor was 8.3%, which was weak. Bonferroni results showed that the mean of pre-plerixafor PB CD34+ cell count was significantly lower than the mean of PB CD34+ cell count at 3, 6, 9, and 12 hours post-plerixafor injection (P-values: before injection/3 h = 0.003, before injection/6 h = 0.001, before injection/9 h & 12 h < 0.001). In addition, pairwise comparisons showed that plerixafor was ineffective over time (Table 2).
| Variables | Simple Regression Analysis | |
|---|---|---|
| Beta (80% CI) | P-Value | |
| Addiction | 0.24 | |
| Yes | -3.57 (-7.51 - 0.35) | 0.24 |
| No (RL) | 0 (-) | - |
| Lenalidomide | 0.99 | |
| Yes | -0.01 (-2.30 - 2.26) | 0.99 |
| No (RL) | 0 (-) | - |
| Radiotherapy | 0.55 | |
| Yes | 1.14 (-1.41 – 3.70) | 0.55 |
| No (RL) | 0 (-) | - |
| Mobilization failure history | 0.78 | |
| Yes | 0.87 (-3.31 - 5.05) | 0.78 |
| No (RL) | 0 (-) | - |
| Peripheral blood CD34+ | ||
| Prior plerixafor | 0.16 (0.06 - 0.25) | 0.03 b |
| Platelete a | ||
| Admit day | 1.79 (-1.69 - 5.27) | 0.50 |
| HSCT day | 5.27 (2.67 - 7.87) | 0.01 b |
| Prior GCSF | 3.73 (-0.18 - 7.65) | 0.22 |
| Prior plerixafor | 0.35 (-1.72 - 2.43) | 0.82 |
| WBC a | ||
| Admit day | 4.79 (0.13 - 9.44) | 0.18 b |
| HSCT day | 0.69 (-0.49 - 1.89) | 0.44 |
| Prior GCSF | 0.37 (-1.99 - 2.74) | 0.83 |
| Prior plerixafor | 0.30 (-1.83 - 2.44) | 0.85 |
Abbreviation: RL, reference level.
a Logarithmic scale.
b Significant at 0.20.
The simple regression analysis of risk factors for CD34+ cell count in apheresis product revealed that pre-plerixafor PB CD34+ cell count, platelet count on HSCT day, and WBC count on admission day were influential. A unit increase in pre-plerixafor PB CD34+ cell count results in a significant increase in the average of CD34+ cell count in apheresis product (Beta = 0.16, 80%CI = (0.06 - 0.5), P-value = 0.03). Besides, a unit increase in logarithmic scale of platelet count on HSCT day and WBC count on admission day results in an increase in the average of CD34+ cell dose in apheresis product by 5 units (Beta = 5.27, 4.79, 80%CIs = (2.67 - 7.87) and (0.13 - 9.44), P-values = 0.01 and 0.18). Influential variables in the simple regression analysis were not significant in multiple analyses (data not shown) (Table 2).
5. Discussion
According to the guideline of the Center for International Blood and Marrow Transplant Research (CIBMTR), in 99% of cases, peripheral blood stem cells are the preferred stem cell source for auto-HSCT (13). This widespread application of peripheral blood stem cells is attributed to their easier collection and faster engraftment (14). However, an adequate dose of CD34+ stem cells cannot be collected in 40% of patients who received anticancer drugs (15).
Plerixafor was approved for stem cell mobilization in lymphoma and myeloma patients in 2009. (16). Plerixafor and G-CSF combination is found to be advantageous as patients who received this mobilization method did not showe adverse effects after chemotherapy. However, several issues about plerixafor treatment have remained unclear, including patient selection criteria, factors affecting mobilization success, and the ideal time interval between plerixafor administration and apheresis (14).
In this study, peripheral blood CD34+ cell counts were enumerated one hour before and then 3, 6, 9 and 12 hours after plerixafor injection. Trends over these periods revealed the peak of CD34+ cell count at 9 hours after drug administration. According to the univariate analysis, basal CD34+ cell count, platelet count on day 0 and before G-CSF treatment, and WBC count on admission day could potentially affect stem cell yield.
In line with preliminary reports, the time interval between drug injection and apheresis onset was 12 hours in this study. Certain studies have also shown that CD34+ cells might reach their maximum level beyond 12 hours, between 16 and 18 hours, after injection (17-19). One possible reason why the extended time interval is critical to consider is probable severe side effects after plerixafor administration. Therefore, drug administration in hospitals could provide more effective and safe treatment (19). Another encouraging factor for an extended interval is plerixafor self-administration by the patient in 12-hour intervals (18). Although there might be no significant difference between hospital and self-administration (20), and it is preferable for patients to be injected by nurses. In our center, patients are admitted before mobilization and injected by qualified nurses. On the other hand, the results of the Lefrere et al. (21) study, have inspired us to figure out the trend of CD34+ cell dose before 12 hours.
In accordance with our study, various researchers have examined the trend of circulating CD34+ cells for 12 hours after plerixafor injection. A study in 2013 showed CD34+ cell count peak within 3 to 6 hours after plerixafor injection in very poor prognosis patients (21). In the present study, however, patients were divided into three groups based on the basal level of CD34+ cells in their peripheral blood: Absolutely poor, relatively poor, and borderline patients. In Lefrere et al. (21) study, all patients had a history of mobilization failure or stem cell collection failure. Devine et al. reported an approximately seven-fold increase in circulating CD34+ cells after 6 hours of plerixafor injection (22). In a study conducted in 2020, a comparison between apheresis products of two groups with 6-hour intervals and 14 to 20-hours intervals indicated higher stem cell dose in the first group (23). A study on sickle cell disease patients illustrated a peak above 80 CD34+/μL in two to three hours after drug administration (24). This result is inconsistent with the results of this study, suggesting the highest concentration of stem cells after 9 hours. It should be noted that healthy volunteers were selected as the population of the study mentioned before (9).
The study by Martino et al. supports the significant effect of platelet count before G-CSF administration in healthy donors on the quantity of collected CD34+ cells (25). In another study, low platelet counts before apheresis in patients with multiple myeloma were significantly associated with poor mobilization outcomes (26). Lanza et al. explored factors influencing the mobilization efficiency with plerixafor, suggesting that basal platelet count is a strong independent predictor of successful mobilization (27). Another research that examined factors affecting apheresis in lymphoma patients reported a significant relationship between platelet levels before mobilization and CD34+ cell dose (28). In a contrary study, platelet levels before stem cell collection did not correlate with stem cell yield. However, patients who required receiving plerixafor had significantly lower platelet levels than other patients (29). In this study, platelet levels were assessed four times: on admission day, before mobilization, before plerixafor injection, and on transplantation day. In this regard, platelet count before G-CSF administration and transplantation day showed a significant relationship with CD34+ cell dose in graft. Nevertheless, further studies are needed to investigate other factors affecting the apheresis outcome and confirm the significant impact of platelet count on day 0 before G-CSF initiation compared to other days.
In a study by Basak et al., no correlation was found between CD34+ cell dose and WBC count on the first day of plerixafor injection (30), which is inconsistent with our results. CD34+ cell count before collection is the most accurate and reliable predictor of mobilization rate (25, 31, 32), which is also confirmed by the present study’s data.
In the current study, data related to lenalidomide and radiotherapy for patients were extracted from patients' records. However, there was no significant relationship between these two factors and apheresis product. It can be inferred that premobilization factors could only give hints and suggest the possibility of poor mobilization (16). Moreover, it can be suggested that plerixafor could potentially weaken the effect of these factors and increase the stem cell dose in apheresis products.
Nevertheless, we did not postpone the initiation of apheresis because the standard time interval between plerixafor and stem cell collection is still considered 12 hours, and changing this time interval requires further consideration, as in some cases, patients could not afford the high cost of this drug. The apheresis procedure for several patients was performed for more than 12 hours (between 13 and 15 hours) with another enumeration at the beginning of stem cell collection, which showed a much higher number of stem cells compared to apheresis after 12 hours (data not shown). Based on this observation and previous reports, possibly the peak of CD34+ cells could occur later. A study by Worel et al. indicated a high-committed cell population in graft after plerixafor mobilization at 6 - 12 hours’ time interval (33). On the contrary, Shi et al. suggested that despite CD34+ cells peak after 12 hours, CD38-/CD34+ cells, with higher priority, reach their peak between 10 and 18 hours (34). This is consistent with findings of a study in patients with sickle cell disease that demonstrated high expression of stemness genes in stem cells mobilized by plerixafor (24). Investigations concerning plerixafor administration time have mostly evaluated the dose of CD34+ cells. Therefore, assessing subpopulation cells might provide insight into the time when CD34+ cells number reach a peak with optimal and favored quantity and quality. In this regard, we can refer to the study of Arcangeli et al., which showed better immune reconstitution after stem cell mobilization with a combination of G-CSF and Plerixafor compared to GCSF alone in xenograft models (35). Accordingly, a more accurate analysis can provide helpful information about the quantity and quality of the mobilized stem cell population.
5.1. Limitations
The main limitations of the current study included a small sample size and no documented adverse effects of Plerixafor administration. The time of apheresis is another difference between our study and previous reports. Stem cell collection starts at 19:00 - 23:00 in our center. It has been reported that performing the apheresis procedure in the evening could significantly increase the stem cell yield in humans (15). However, there are not enough case-control studies to compare the frequency of harvested CD34+ cells in the mornings and evenings, and this could represent a notable distinction between our study and previous ones.
5.2. Conclusions
Based on the differences between the results of previous studies, regular peripheral blood stem cell enumeration could draw the kinetics of each patient. Additional studies with a larger sample size are required to provide reliable insights into the peak of CD34+ cells in peripheral blood due to the high cost of the Plerixafor drug.