1. Background
Epidemiology is the knowledge of disease distribution in a population and is essential for the prevention, detection, and treatment of them. Any national, regional, or global planning or monitoring is based on epidemiologic data.
Breast cancer is the most prevalent cancer in Iran and worldwide. More than 90% of breast cancers are adenocarcinoma, which arises from epithelial tissue. On the other hand, breast sarcomas are non-epithelial malignancies that arise from connective tissue of the breast (1, 2). They are a heterogeneous group of diseases and account for less than 1% of breast malignancies (2, 3). Phyllodes tumor is composed of both malignant connective tissue and benign epithelial component; therefore, in some breast sarcoma studies phyllodes, the tumor is excluded. However, as its behavior and management are similar to other breast sarcomas, many authors consider it a subtype of sarcoma (3-5).
The annual incidence rate of breast sarcoma has been reported at 4.48 cases per million women in the United States (3-6). Due to its rarity, there are few epidemiologic studies on breast sarcoma and most published articles are case reports or small case series.
This disease is most common in the 5th and 6th decades of life (4). However, the peak incidence of malignant phyllodes tumor in women is between 35 and 55 years of age (7). Breast sarcoma is rare in men. Only 1.5% of breast sarcoma cases are male and the rest are female (3).
In this report, the age-specific incidence rate of breast sarcoma, as well as the age-standardized incidence rate (ASR) for each morphologic subtype and its geographic distribution have been studied. To our best knowledge, this is the first epidemiologic study on breast sarcoma in Iran and can provide essential information for further studies.
2. Methods
The data of this study were collected from the Iran National Cancer Registry (INCR) supervised by the Iranian Ministry of Health and Medical Education. All patients registered as “breast sarcoma” from March 20th, 2009 to March 20th, 2014 were enrolled in this study. The registry is mandatory and conducted based on ICD-O 3 guidelines (8). It was a requirement for hospitals, clinics, and pathology laboratories to report patient information to the INCR. The information includes patients’ national ID, date of birth, gender, pathologic subtype, grade, and the regional center. Breast cancer was identified by their histology as phyllodes tumor, angiosarcoma, fibrosarcoma, liposarcoma, malignant fibrous histiocytoma, rhabdomyosarcoma, leiomyosarcoma, osteosarcoma, and not otherwise specified (NOS). The country is divided into 12 geographic regions, including Ahwaz, Hamedan, Isfahan, Kerman, Kermanshah, Mashhad, Qom, Rasht, Sari, Shiraz, Tabriz, and Tehran. In the zoning of Iran’s provinces, proximity, geographical location, and commonalities were taken into consideration. Patients are registered based on their living area region.
There were potential issues with the data, such as multiple records for some patients. In addition, an incorrect morphology may have been recorded for a topography associated with breast cancer. Patients’ birth dates and ages did not match in some cases. Therefore, data quality needs to be assessed. Accordingly, multiple steps were involved in the INCR’s extraction and cleanup of the analyzed dataset. In the first step, the consistency of the topographical and morphological information of the patients was assessed. The data re-examined in case of any inconsistencies, and if the data were inaccurate, the subject excluded from the study. As a next step, the accuracy of patients’ birthdays and diagnosis dates was checked. Any inaccurate information was modified or removed. All patients with the same first name, surname, and ID number were considered duplicate cases and excluded from the study.
Population data were extracted from the national “Population and Housing Census” in 2006, 2011, and 2016 and the population of other years were calculated based on the official population growth rate, declared by the “Statistical Center of Iran” (9). During the course of the study, the updated data could not be used due to the time gap between cancer data collection and patient medical records. Therefore, the latest national data were used.
ASR and 95% CI were calculated in 5-year age groups, using the new World Health Organization (WHO) standard population as follows:
and
and
Where Zα/2 is a standardized normal deviation, ai is the crude incidence rate in each age category (age-specific incidence rate), and wi is the new WHO standard population (10, 11). ASR was calculated for each year, geographic region, morphology subtype, and grade. R (version 4.0.2), and SPSS (version 26) was used for all analyses. P-values less than 0.05 were considered statistically significant.
3. Results
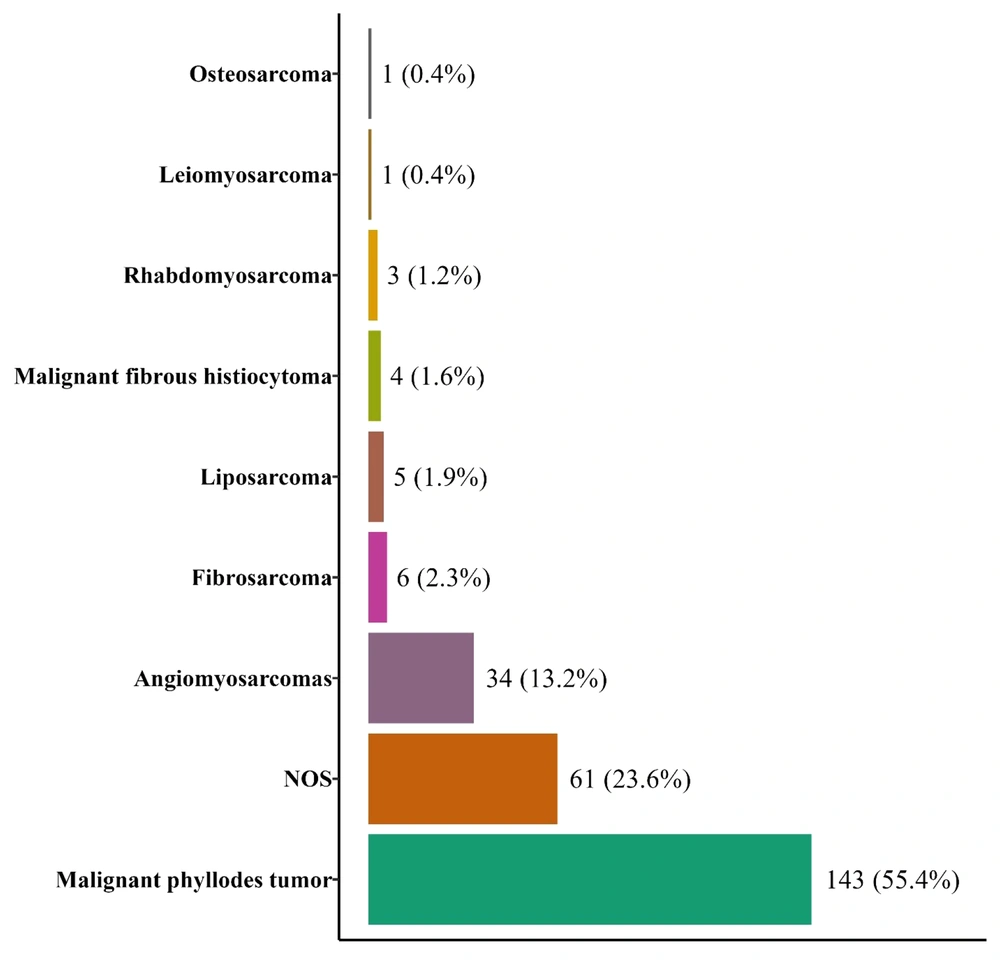
A total number of 258 breast sarcoma patients were diagnosed from March 20th, 2009 to March 20th, 2014, of whom 143 cases had malignant phyllodes tumors. Others included angiosarcomas (12), fibrosarcoma (6), liposarcoma (5), malignant fibrous histiocytoma (4), rhabdomyosarcoma (3), leiomyosarcoma (1), and osteosarcoma (1). Sixty-one cases were classified as NOS (Figure 1). ASRs (95% CI) were calculated for each morphologic subtype as well as the total number of patients (Table 1). Accordingly, overall ASR was 0.60 (95% CI: 0.52 - 0.67) per million person-years with 0.03 (0.01 - 0.06) in male and 1.17 (1.02 - 1.32) in females. The highest ASR per million person-years was found for phyllodes tumor malignant (0.33; 95% CI: 0.28, 0.39), NOS (0.15; 95% CI: 0.11, 0.19) and angiosarcoma (0.08; 95% CI: 0.05, 0.10). Seven out of 258 cases (2.7%) were male, including 3 phyllodes tumors, 1 liposarcoma, and 3 NOS type.
| Histology Subtype | ASR (95% CI) |
|---|---|
| Phyllodes tumor malignant | 0.33 (0.28 - 0.39) |
| NOS | 0.15 (0.11 - 0.19) |
| Angiosarcoma | 0.08 (0.05 - 0.10) |
| Liposarcoma | 0.01 (0.00 - 0.03) |
| Fibrosarcoma | 0.01 (0.00 - 0.02) |
| Malignant fibrous histiocytoma | 0.01 (0.00 - 0.02) |
| Rhabdomyosarcoma | 0.01 (0.00 - 0.01) |
| Osteosarcoma | 0.00 (0.00 - 0.01) |
| Leiomyosarcoma | 0.00 (0.00 - 0.01) |
| Total | 0.60 (0.52 - 0.67) |
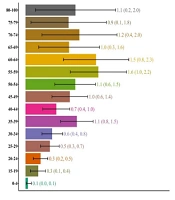
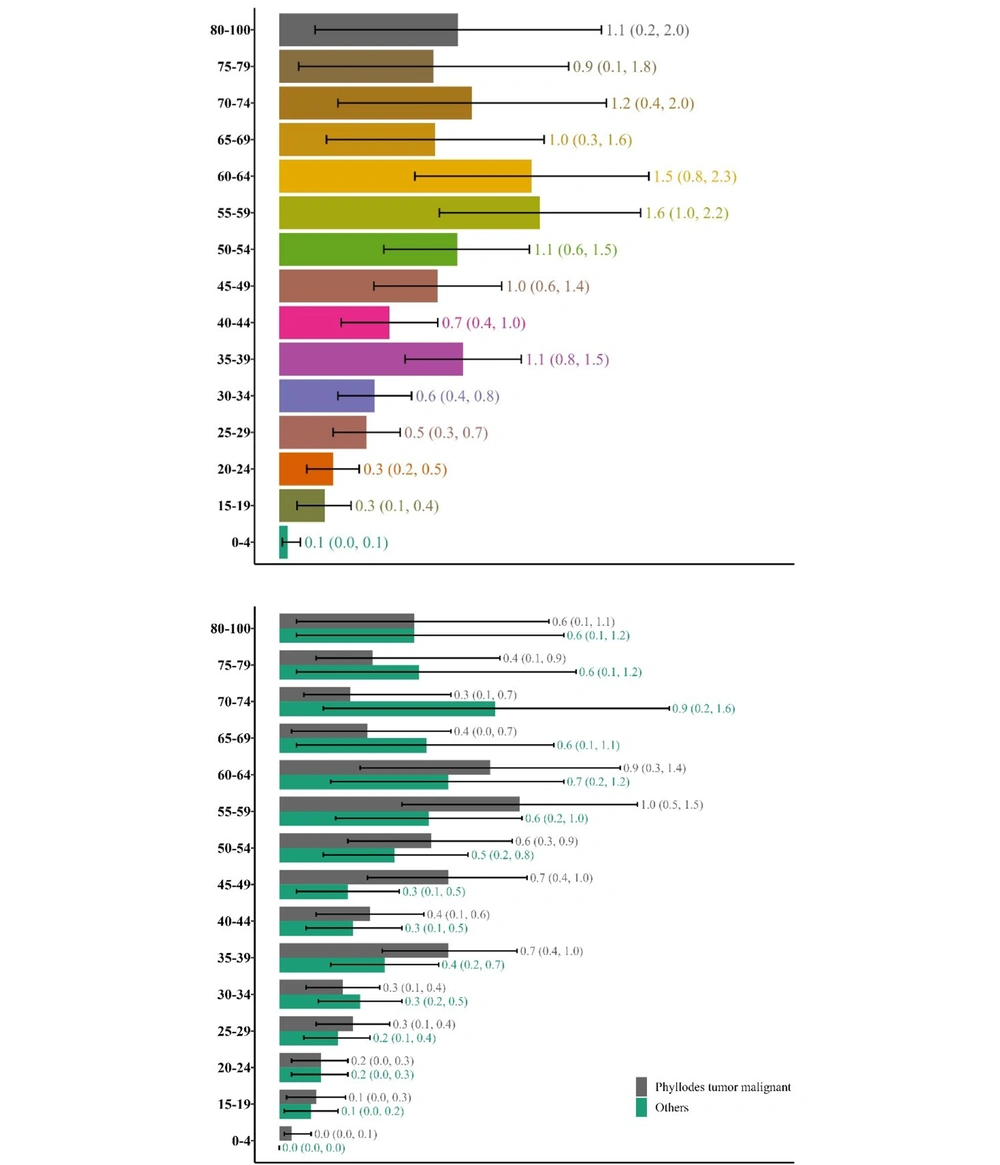
Phyllodes tumors are seen in more than half of the cases and it happens in younger patients compared to other sarcomas. In this study, the age-specific incidence rate has been calculated for phyllodes tumors and other sarcomas both separately and together so that the numbers can be compared with other publications whether they have included phyllodes tumors in their study or not. For malignant phyllodes tumors, the highest age-specific incidence rate per million was in the 55- to 59-year-old group (1.0; 95% CI: 0.5, 1.5) while for other sarcomas, it was in the 70- to 74-year-old group (0.9; 95% CI: 0.2, 1.6). The age-specific incidence rate was calculated for all sarcomas together as well and the highest incidence for all sarcomas was in the 55- to 59-year-old group (1.6; 95% CI: 1.0, 2.2) (Figure 2).
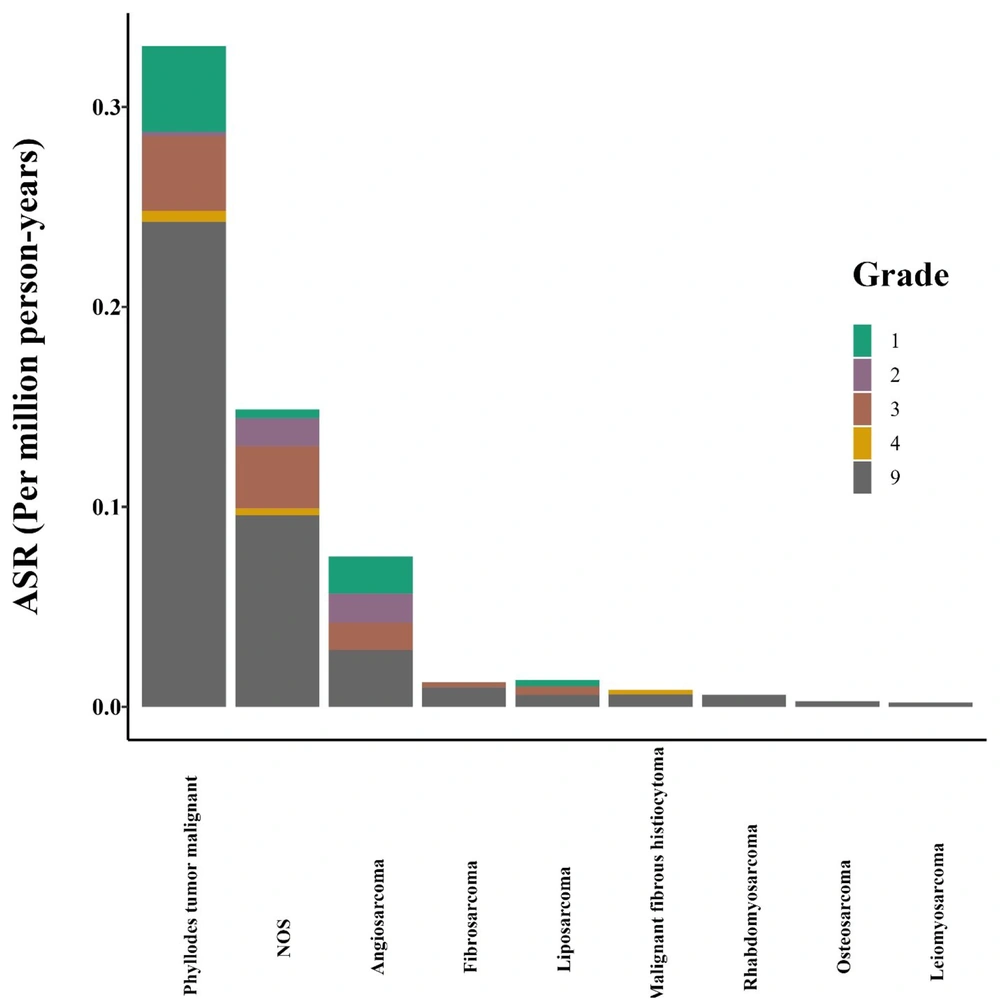
Grading was based on the “6-digit code for histological grading and differentiation” (Table 2) (13). The frequency (%) of grades 1, 2, 3, 4, and 9 was 31 (12.0%), 14 (5.4%), 40 (15.5%), 4 (1.6%) and 169 cases (65.5%), respectively. ASR for each morphologic grade was calculated as well (Figure 3 and Appendix 1 in the Supplementary File).
| Digit | Grade | Level of Differentiation |
|---|---|---|
| 1 | Grade I | Well differentiated |
| Differentiated | ||
| NOS | ||
| 2 | Grade II | Moderately differentiated |
| Moderately well-differentiated | ||
| Intermediate differentiated | ||
| 3 | Grade III | Poorly differentiated |
| 4 | Grade IV | Undifferentiated |
| Anaplastic | ||
| 9 | Grade or differentiation not determined, not stated or not applicable |
The highest number of patients was registered in Tehran, followed by Ahwaz and Isfahan from 2009 to 2014. Appendix 2 and 3 in the Supplementary File showed the frequency of breast cancer in each province and each area (Appendix 2, 3 and 4 in the Supplementary File).
4. Discussion
Breast sarcoma is a rare and aggressive cancer. The usual presentation is a unilateral painless fast-growing mass (14). Skin and nipple changes or axillary lymphadenopathy are not common. They have no pathognomonic imaging features and mammographic and sonographic findings are non-specific (15). Diagnosis is usually made by core needle biopsy.
There are different subtypes of breast sarcoma. Our study revealed that after malignant phyllodes tumor, the most common subtypes are sarcoma NOS type, angiosarcoma, and fibrosarcoma, respectively. The most common mesenchymal malignancies of the breast are phyllodes tumor, followed by angiosarcoma (2, 3, 16-20). Yin et al. utilized a US population database to analyze a large series of women diagnosed with primary breast sarcoma, excluding phyllodes tumor (16). In their study, angiosarcomas, sarcoma NOS, spindle cell sarcoma, and fibrosarcoma were the most prevalent (16).
Some authors do not include malignant phyllodes tumors in their study. Wang et al. conducted a retrospective review of 35 cases with primary breast sarcoma (PBS) and 70 cases with malignant phyllodes tumor (MPT) (21). They concluded that MPT and PBS may be considered together because these two diseases have similar behavior, disease-free survival (DFS), and overall survival (OS). Moreover, both diseases should be treated with the same strategy (5, 21). Compared to other sarcomas, phyllodes tumor occurs in younger women. As this subtype of sarcoma composed 55.4% of our cases that could affect the average age, ASR was calculated both for all cases together and for phyllodes and other cases separately. This study revealed that the highest incidence rate for malignant phyllodes tumor in Iran is in 55- to 59-year-old women, which is higher than the literature (35 to 55 years) (7, 17, 22). Excluding phyllodes tumor, the highest incidence rate for other sarcomas was between the 70- to 74-year-old group. This is higher than the literature as well (the 5th and 6th decades) (4, 16). Patients with primary breast sarcoma have a lower age compared to the secondary breast sarcoma patients (23, 24). However, if all cases are calculated together, the highest ASR will be in the 55- to 59-year-old group that is compatible with the literature.
Pediatric breast cancer (19 years old and younger) is rare and comprises less than 0.1% of all breast cancers (25, 26). About 5% of phyllodes tumors occur in girls younger than 20 years. Low-grade forms of sarcoma have been reported in children in their second decade of life (25-27). In a population-based study in the United States, it was shown that 35.1% of all pediatric breast malignancies were fibroepithelial tumors and 14.2% were sarcoma (21). In this study, 13 patients were 19 years old or younger consisting of 5% of all patients. Two of these patients were in the age group of 0 - 4 and both of them had malignant phyllodes tumor.
In data compiled from the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute, the annual incidence of breast sarcomas was 4.48 cases per million women in the United States (3-5). May and Stroup used the SEER data from 1973 to 1986 and calculated the annual incidence rate of 4.48 cases per million women (28). In this study, the annual incidence rate in Iran was 1.17 per million, which is lower than in western countries.
Al-Benna et al. reviewed major sarcoma series and found that only 1.5% of breast sarcomas occur in men (3). In another review conducted by Lim et al., 2.4% of patients were male (29). However, our study revealed that 2.7% of breast sarcoma patients in Iran are male. We have no clear explanation that why while the overall incidence of breast sarcoma is lower in Iran, it has a higher incidence in men.
The ASR was highest in Ahwaz. The reason is unknown and could be an interesting field for further studies. External beam radiation, chronic lymphedema, hereditary diseases like Neurofibromatosis or Li-Fraumeni-syndrome, and long-standing contact with vinyl chloride are known risk factors for developing sarcoma (3, 30). However, the risk factors for developing breast sarcoma are mostly unknown and most the sarcomas have no demonstrable cause (31). In this study, most of the sarcoma was grade 3, which is compatible with other studies that high-grade sarcoma has been more prevalent.
In this timeframe, 11 cases of carcinosarcoma were registered as well. This is a rare neoplasm that accounts for 0.08 to 0.2% of all breast malignancies (32). This tumor is composed of both malignant epithelial (carcinomatous) components and malignant non-epithelial (sarcomatous) components with a clear-cut boundary between them (32-34). Carcinosarcoma, as defined by WHO, is a subtype of metaplastic carcinoma (12), and their behavior and treatment strategy is more like a carcinoma; therefore, they were not included in this study.
A faster and more accurate diagnosis, as well as a more comprehensive registration, are essential for improving cancer registration. Cancer centers should be able to provide everyone with rapid identification and accurate diagnosis of cancer at an affordable price. Moreover, software and hardware resources should be encouraged to register cancer data comprehensively and precisely. Furthermore, individuals and staff who will be recording data should be well-informed and fully aware of the importance of accurate data recording.
4.1. Conclusions
Breast sarcoma is a rare disease with remarkable heterogeneity. Compared to western countries, Iran has a lower incidence of breast sarcoma in women, a higher incidence rate in men, and older onset age. As in other countries, malignant phyllodes tumors and angiosarcomas are the most common subtypes. In addition, breast sarcoma incidence rates in different grades are similar across countries. Geographically, it is more common in Ahwaz. Additional research in this area may be warranted.