1. Background
Angiogenesis is critical for tumor growth and metastatic spread (1), which is influenced by various biomolecules such as vascular endothelial growth factor (VEGF) (2). Once tumors grow to 1 - 2 mm in diameter, the tumor's inner cells become hypoxic and increase VEGF expression (3).
VEGF has two main vascular endothelial growth factor receptors (VEGFR), VEGFR1 and 2, which are involved in angiogenesis. The binding of VEGF to its receptor activates signaling pathways resulting in the increased expression of matrix metalloproteinase (MMPs) and cyclin D1, change in cell membrane integrin profile, reduction in vascular endothelial (VE)-cadherin connections, and therefore an increase in invasion, migration, and cell proliferation and ultimately increased angiogenesis (4).
Chemotherapy is one of the main cancer treatments that can also be applied as the tumor progression-preventing therapy through angiogenesis suppression, but these drugs have adverse effects on normal tissues (5-7). Hence, the studies on the decreased usage doses and subsequently the side effects of chemotherapy drugs have focused on combining these drugs withnatural compounds (8).
5-Fluorouracil (5-FU) is a chemotherapy drug employed to treat various cancers, such as breast, cervical, and gastrointestinal cancer (9). It has been shown that 5-FU inhibits angiogenesis and migration by suppressing the expression of angiogenic factors, including VEGF and MCP-1 (10).
Quercetin (Que) is a natural polyphenolic compound found in fruits and vegetables, such as onions, apples, etc (11). it is reported that Que act as an inducer of apoptosis and cell cycle arrest and an inhibitor of metastasis, and angiogenesis in cancers (12). By targeting the VEGFR-2 expression and its signaling pathway, Que attenuates the angiogenesis (13).
2. Objectives
Que can be utilized as an enhancer of the anti-angiogenic effect combined with chemo drugs. In addition, Que enhances the anti-angiogenic effect of these drugs, usage dose, and the side effects of these drugs. In the present study, we evaluated the effects of 5-FU and Que alone and in combination on the viability, migration, VEGFR1 and VEGFR2 gene expression of human umbilical vein endothelial cells (HUVECs), and in vivo neovascularization.
3. Methods
3.1. Cell Culture
Human umbilical vein endothelial cells (HUVECs) were provided by Pasteur Institute (Tehran, Iran). HUVECs were cultured in Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM-F12 medium) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (all reagents were purchased from Bio-Idea Tehran, Iran). The cells were incubated at 37°C with 95% humidity and 5% CO2 (14).
3.2. Preparation of Treatments
5-FU at 50 mg/mL was obtained from Ebewe Pharmacy (Unterach, Austria). By adding the right amount of drug as a supplement to the medium, we obtained the desired concentration. Que was purchased from Sigma-Aldrich (St. Louis, Missouri, United States) and dissolved in dimethylsulfoxide (DMSO, Bio-Idea) and the stock solution was prepared at 200 µM, which was diluted with the medium to prepare different concentrations. The concentration of DMSO in the cell culture medium was < 0.1%. Thus, it failed to affect any of the cell functions.
3.3. The Cellular Metabolic Activity (the MTT Assay)
The MTT assay was applied to measure the cell viability in culture; for this purpose, 6 × 103 (HUVECs) cells per well were seeded in 96-well plates and incubated for 24 h. Afterwards the cells were treated with different concentrations of 5-FU (2.5, 5, 10, 20, 40, 80, and 160 μM), Que (10, 40, 70, 100, 130 and 160 μM), and various combination states with 2.5 and 5 μM of 5-FU plus 70, 100, and 130 μM of Que for 24 and 48 h. Subsequently, the medium was replaced by the use of MTT solution at the final concentration of 0.5 mg/mL and the cells were incubated for 4h in a dark place. The MTT solution was then removed and 150 μL DMSO (Merck, Darmstadt, Germany) was added and shacked for 15 minutes. The plates were read with a microplate reader (BioTek ELx800 Winooski, Vermont, United States) at a wavelength of 570 nm. IC50 values were calculated via the computer software Graphpad Prism 8 (La Jolla, CA). The assay was performed in triplicate with 4 replicates per sample (14).
3.4. Wound-Healing Migration Assay
Cell migration was evaluated via wound healing assay. To this end, 200,000 cells per wells were seeded in 6-well plates and incubated at 37°C to reach a 90% confluency. Afterwards the cells were scratched utilizing the sterile yellow tip and washed with PBS to remove debris and treated with 5 μM 5-FU, 130 μM Que, and their combination. The wells were photographed at 0, 24, and 48 h in random microscopic zones, andImage J software (Bethesda, USA) was used to assess the width of the scratches. Subsequently, the percentage of wound closure was calculated according to the following formula (15):
3.5. Real-time RT-PCR
The total RNA was extracted with a Hybrid-R RNA isolation kit from the harvested cells according to the manufacturer’s instructions (GeneAll, Songpa-gu, Seoul, South Korea). cDNA was synthesized from the extracted RNAs using the cDNA synthesis kit based on the manufacturer’s instructions (Yekta Tajhiz Azma, Iran). In brief, 2 μL of cDNA was amplified in each 20 μL PCR reaction mix containing 10 μL of 2x SYBR Green Master Mix (Yekta Tajhiz Azma, Iran), 1 μL of each 10 μM forward and reverse primers and 6 μL DEPC water. HPRT was preferred as the internal reference. The results were analyzed utilizing an Applied Biosystem with software version 2.3 (StepOneTM, USA). The reaction conditions were as follows: 94°C for 3 min, 94°C for 40 sec, 59°C for 30 sec, 72°C for 30 sec for 40 cycles, and 72°C for 5 min. The primers were designed using Allele ID software version 7.5 (Premier Biosoft, USA) and their sequences are represented in Table 1.
| Gene | Seq. (5-3) | |
|---|---|---|
| VEGFR1 | F | CTGCTACCACTCCCTTGA |
| R | TCCACTCCTTACACGACAA | |
| VEGFR2 | F | TGGAGGAGGAGGAAGTAT |
| R | CGTCTGGTTGTCATCTGG | |
| HPRT | F | GACCAGTCAACAGGGGACAT |
| R | CCTGACCAAGGAAAGCAAAG | |
Abbreviation: VEGFR, vascular endothelial growth factor receptors.
3.6. Chick Chorioallantoic Membrane (CAM) Assay
To evaluate the in vivo angiogenesis, CAM assay was performed. The fertilized chicken eggs (purchased from the Department of Poultry, School of Veterinary Medicine, Shiraz University) were incubated at 37°C and 60% humidity and randomly were divided into 4 groups: (1) control, (2) 5 μM 5-FU, (3) 130 μM Que, and (4) 5 μM 5-FU plus 130 μM Que (n = 4 per group). Briefly, on the second day of incubation, a 1-cm2 square window was made at the top of the live eggs and 2 mL of albumin was aspirated on the opposite side. On the fifth day, the CAM was developed; hence, the sterile methylcellulose discs loaded with the drug were applied to CAMs. The drug-treated eggs were incubated at 37°C and 60% humidity for 48 h. Subsequently, the CAM tissues under the filter discs were removed, washed in phosphate-buffered saline (PBS), and fixed with formaldehyde 4%. Lastly, the images (150X) were taken by the use of a stereomicroscope (Leica Zoom 2000). The images were analyzed using online Wimasis Image Analysis Software. The number of total branch points and total vessel network lengths were utilized as indicators of angiogenesis (16).
3.7. Statistical Analysis
Values were presented as the mean ± S.E.M and the results were analyzed using SPSS software version 22. The data were compared by use of the one-way analysis of variance (ANOVA) and LSD analysis. P < 0.05 was considered to indicate a statistically significant difference.
4. Results
4.1. Effects of 5-FU, Que, and Their Combination on Cell Viability
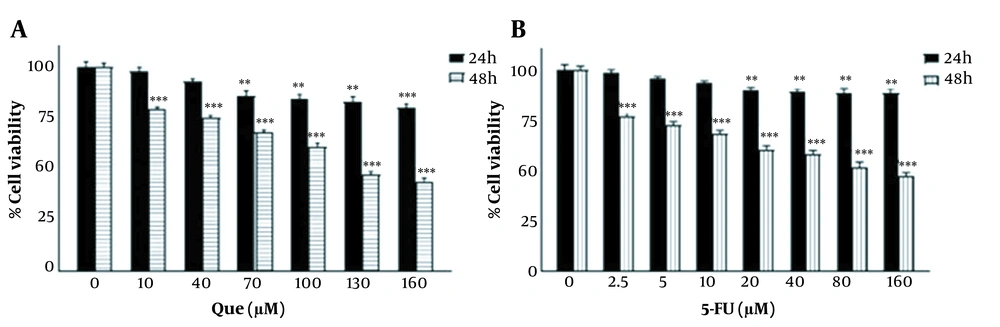
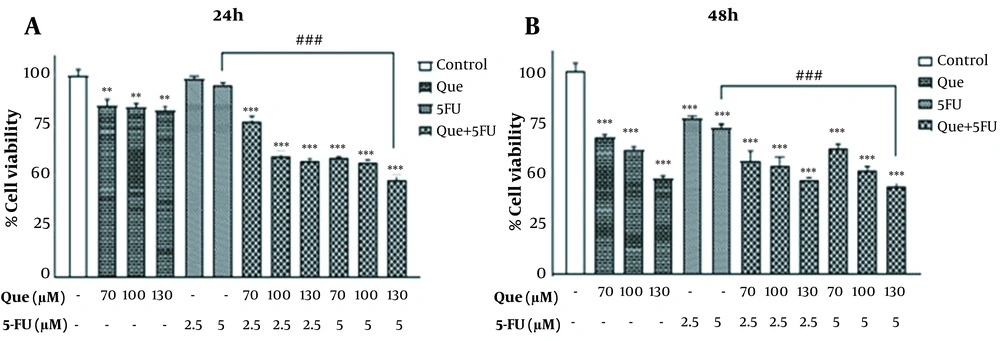
The MTT assay was applied to determine the IC50 values, choose the appropriate concentration of drugs for the combination, and eventually evaluate the combined effect. As shown in Figure 1A, different concentrations of Que reduced the cell viability compared to the control untreated sample. This reduction was dose-dependent and at 160 μM, it was revealing a maximum inhibition of 55% after 48 h of incubation. Different concentrations of 5-FU also decreased the cell viability in a dose-dependent manner and the maximum inhibition of 53% was observed at 160 μM after 48h of treatment (Figure 1B). The IC50 values for Que and 5-FU at 48h were 130 μM and 160 μM, respectively. In the next step, according to the results of alone treatments, the 6 combination states of 5-FU (2.5 and 5 μM), with a few effects on the growth inhibition of HUVECs and Que (130, 70, and 100 μM) were employed for treatment. As shown in Figure 2, all the combination states revealed a significant reduction in the cell viability at 24h and 48h compared to 5-FU alone treatment. These results indicated that Que significantly enhanced the effect of 5-FU on the cell viability. The highest growth inhibition was observed in the combination of 130 μM Que and 5 μM 5‑FU; therefore, this combination state was applied in the rest of the experiments. The comparison of the combination treatment of 24 with 48 h implied that the reduction in cell viability was also time-dependent, in addition to being dose-dependent.
Effects of Que and 5-FU on cell viability of HUVECs. A, HUVECs were treated with different concentrations of Que for 24 and 48 h. Cell viability was assessed using MTT assay; B, HUVECs were treated with the different concentrations of 5-FU for 24 and 48 h. The cell viability was assessed utilizing the MTT assay. The results are shown as the mean ± S.E.M of 3 independent experiments (**P < 0.01; ***P < 0.001 compared with the control).
Effect of Que and 5-FU combinations on the cell viability of HUVECs. A, Viability of HUVECs after treatment with Que (70, 100, and 130 μM) combined with 5-FU (2.5 and 5 μM) for 24 h was measured using MTT assay; B, Viability of HUVECs after treatment with Que (70, 100, and 130 μM) combined with 5-FU (2.5 and 5 μM) for 48h was measured utilizing MTT assay. The results are shown as the mean ± S.E.M of 3 independent experiments (**P < 0.01 and ***P < 0.001 compared with the control, ###P < 0.001 compared with 5-FU-alone group).
4.2. Effects of 5-FU, Que, and Their Combination on Cell Migration
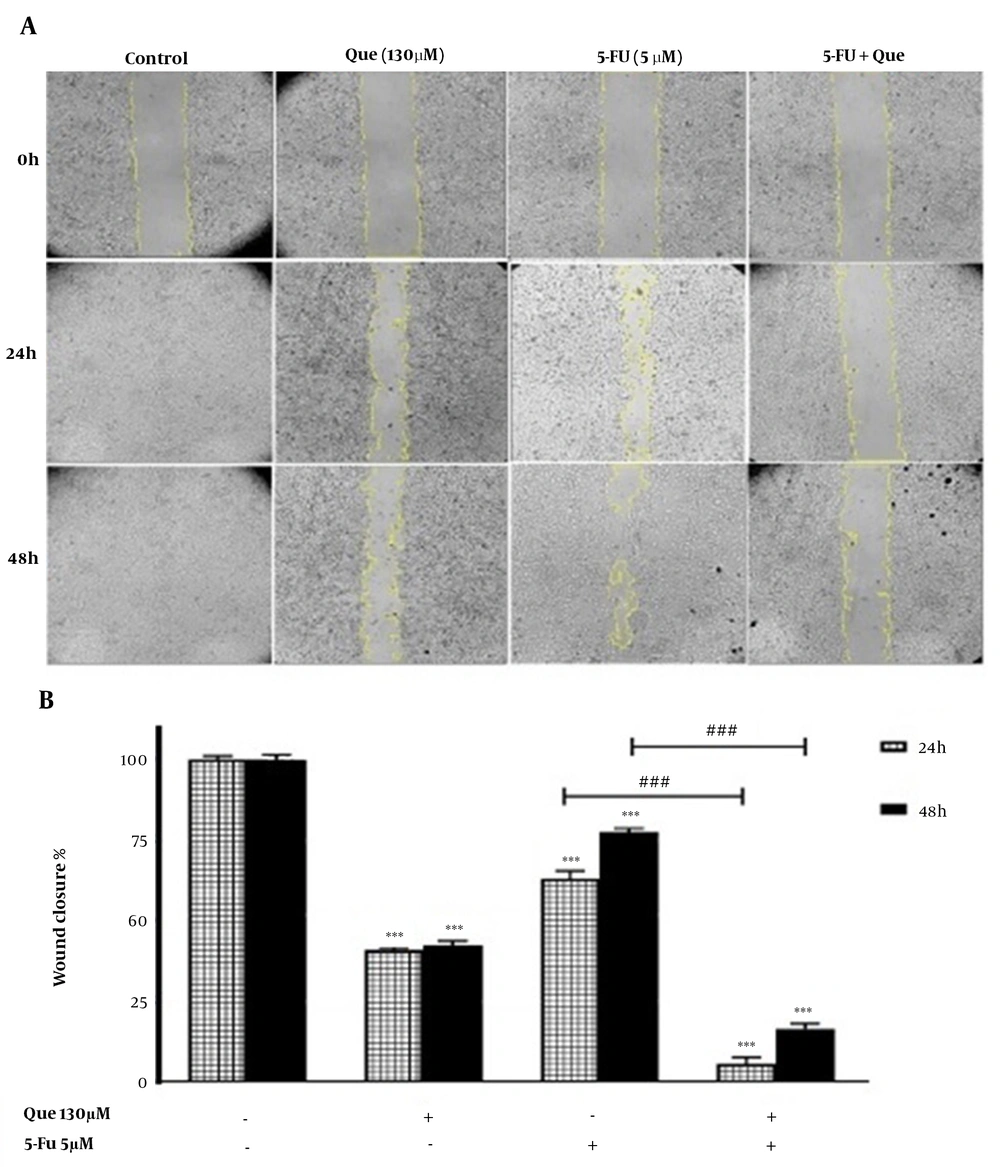
The migration rate of the cells was assessed using a wound-healing assay. The anti-migration effects of 130 μM Que, 5 μM 5‑FU, and their combination at 24 and 48 h are depicted in Figure 3. The results revealed that Que significantly inhibited cancer cell migration and the percentage of wound closure, as an indicator of cell migration, after 24 and 48 h was 40.1% and 42.6%, respectively. Moreover, in 5-FU-treated cells, the percentage of wound closure after 24 and 48 h was 62.9% and 77.3%, respectively. The combination of Que and 5‑FU compared to 5 μM 5‑FU alone significantly inhibited the migration of HUVECs and the width of the scratch was almost similar to that of at 0h. The percentage of wound closure in the combined treatment after 24 and 48 h was 5.5% and 16.3%, respectively. These results indicated that Que significantly increased the anti-migration effect of 5-FU.
Wound healing assay of HUVECs treated with 5-FU and Que. A, Image of HUVECs migration following treatment with Que (130 μM), 5FU(5 μM), and their combination for 0, 24, and 48 h; B, Quantitative analysis of the anti-migration effect of Que (130 μM), 5-FU (5 μM), and their combination for 24 and 48 h. The results are shown as the mean ± S.E.M of three independent experiments (***P < 0.001 compared with the control, ###P < 0.001 compared with 5-FU-alone group).
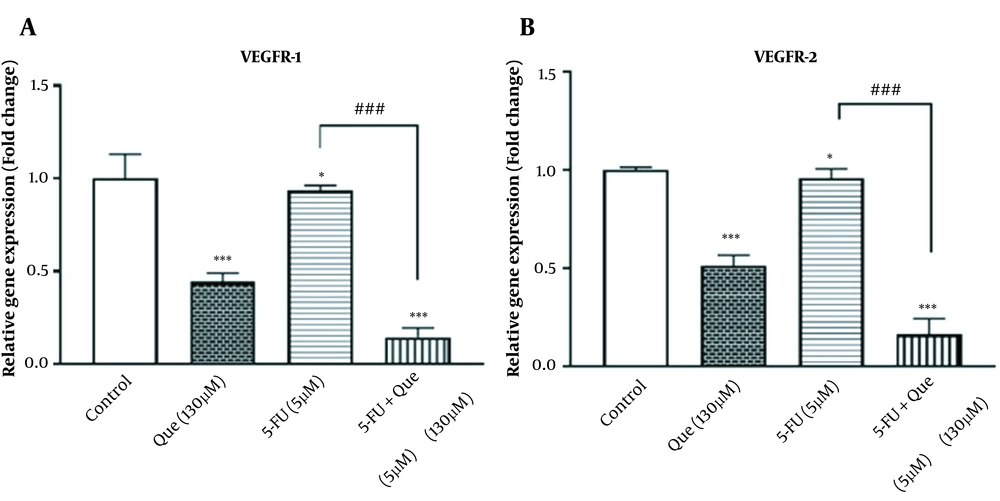
4.3. Effects of 5-FU, Que, and Their Combination on the Gene Expression of VEGFR-1 and VEGFR-2
The effects of alone and combination treatment of Que and 5-FU on the gene expression of VEGFR1 and VEGFR2 were assessed. The effects of Que and 5-FU on the VEGFR-1 and VEGFR-2 genes expression were assessed alone and in combination. As shown in Figures 4A and B, 5 μM 5-FU significantly reduced the gene expression of VEGFR1 and VEGFR2 to 0.93 and 0.95 fold, respectively. Que significantly decreased the gene expression of VEGFR1 and VEGFR2 to 0.44 and 0.51 fold, respectively. However, the combination of Que with 5-FU could significantly reduce the gene expression of VEGFR1 and VEGFR2 compared to 5-FU alone by 0.14 and 0.16 fold, respectively.
Effect of Que, 5-FU, and their combination on the VEGFR-1 and VEGFR-2 gene expression in HUVECs. Que, 5-FU, and their combination reduced the gene expression of VEGFR-1 and VEGFR-2. A, Expression of VEGFR-1 gene was evaluated in HUVECs untreated control cells, treated cells with Que (130 μM), 5-FU (5 μM), and the combination of Que + 5-FU employing quantitative real-time PCR; B, Expression of VEGFR-2 gene was evaluated in HUVECs untreated control cells, treated cells with Que (130 μM), 5-FU (5 μM), and the combination of Que + 5-FU using quantitative real-time PCR. The results are shown as the mean ± S.E.M of 3 independent experiments (*P < 0.05 and ***P < 0.001 compared with the control, ###P < 0.001 compared with 5-FU-alone group).
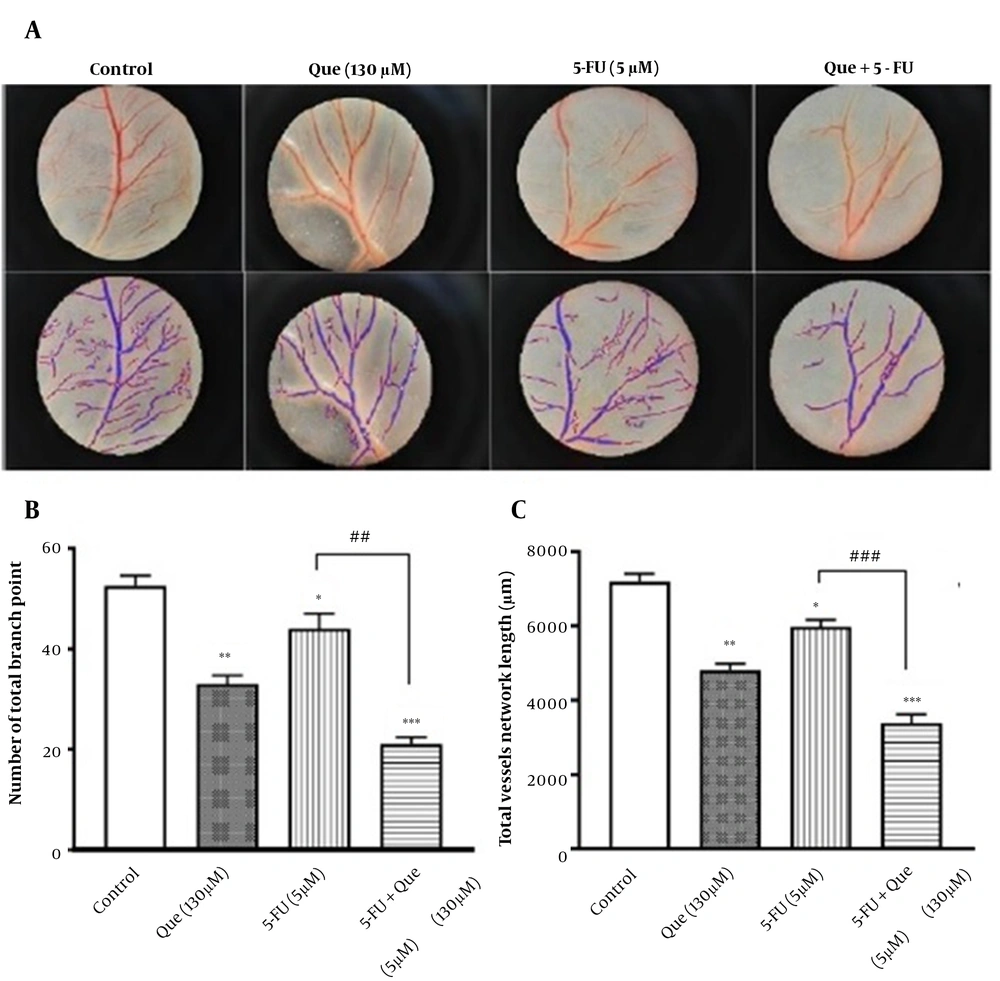
4.4. Effects of 5-FU, Que, and Their Combination on CAM Angiogenesis
CAM assay was applied to investigate the anti-angiogenic effect of the treatments. In Figure 5A, the angiogenesis of CAM with and without treatment is depicted in the first row and their analysis through the Wimasis software is shown in the second row. As shown in Figure 5B, the number of total branch points and total vessel network lengths as the 2 indexes of angiogenesis revealed a similar pattern following the treatments. It means that these indexes significantly decreased with Que and 5-FU compared to the control and the decrease was significant in their combination compared to 5-FU alone.
Effect of Que, 5-FU, and their combination on the in vivo angiogenesis. A, The first row shows the images of the angiogenesis of CAM following the treatment with Que (130 μM), 5-FU (5 μM), and their combination (n = 4 eggs per group); B, The second row is the images of software analysis of each mentioned condition quantitative analysis of the anti-angiogenic effect of Que (130μM), 5-FU (5μM), and their combination based on the number of the total branch point; C, Quantitative analysis of the anti-angiogenic effect of Que (130 μM), 5-FU (5 μM), and their combination based on total vessels network length. Results are presented as the mean ± S.E.M of at least 3 independent experiments (*P < 0.05, **P < 0.01, and ***P < 0.001 compared with control; ###P < 0.001 compared with 5-FU-alone group).
5. Discussion
Angiogenesis is necessary for the growth and metastases of tumors (1); thus, the inhibition of angiogenesis could be a promising therapeutic strategy to prevent cancer progression. Invasion, migration, proliferation, and tube formation of endothelial cells are the essential steps in the angiogenic cascade, targeting anti-angiogenic strategies to prevent new vessel formation (17). 5-FU is one of the key chemo drugs to treat various cancers, including breast and colorectal cancer, which has an anti-angiogenic along with a cytotoxic effect. However, the long-term application of 5-FU causes drug resistance in the cancer cells and the development of destructive effects on the normal tissues (5). Therefore, finding a way to reduce the dose of chemotherapy agents while maintaining or enhancing their therapeutic effects has become an interesting research topic (18). In this study, Que as a natural compound with anti-angiogenic effects was employed to enhance the anti-angiogenic effect of 5-FU. Our results indicated that Que promotes the effect of 5-FU on the growth inhibition, migration, and new vessel formation of the endothelial cells.
To evaluate the effect of Que, 5-FU, and their combination on the endothelial cell viability, the MTT assay was performed. The results indicated that Que significantly enhanced the effect of 5-FU on the cell viability. The combination state significantly reduced the endothelial cell viability compared to 5-FU alone. The highest growth inhibition was observed in the combination of 130 μM Que and 5 μM 5‑FU; so, the rest of the experiments were followed by this combination state. The comparison of the combination treatment of 24 with 48 h implied that the reduction in cell viability was also time-dependent, in addition to being dose-dependent. These results are in line with those of the other study which revealed the combination of 5-FU with interferon-alpha and methylglyoxal (MG) enhances its effect on growth inhibition of endothelial cells and MCF7, respectively (19). in that study combination of 60 μM 5-FU with 0.25 mM MG decreased cell viability by 25% at 24 h, but we reach to 40% reduction in cell viability with the combination of 130 μM Que plus 5 μM 5‑FU at the same time.
The wound-healing assay was utilized to determine the migration rate. The results revealed that the percentage of wound closure is significantly decreased in the Que and 5-FU alone treatment. In the combined application, the decrease significantly was more than the 5-FU alone treatment. The results proved that Que enhances the anti-migration effect of 5-FU on HUVEC. These observations were time-dependent, thereby the effects being more potentiated at 48 h than at 24 h. The results of this part of the study were in agreement with the previous reports that showed enhanced the anti-migration effect of 5-FU in combination with RU-A1, calcium supplementation, and resveratrol on the hepatocellular carcinoma (HCC) and colorectal cancer (CRC), respectively (20, 21). In addition, it also showed that the combination of 1 nM 5-FU with 5μM resveratrol decreased migration by 5% after 10 days, but we reach to 30% decrease in cell migration with the combination of 130 μM Que plus 5 μM 5‑FU at 48 h. The difference in the percentage of migration inhibition may be somewhat related to using the different methods.
Related to VEGFRs, we revealed that the alone treatment of Que and 5-FU significantly decreased the gene expression of both receptors, especially VEGFR1, compared to the control group. However, the gene expression of both receptors showed a significant decrease following the combination treatment compared to the treatment of each drug alone. These results are in accordance with those of other studies showing the decreased expression of VEGFRs with Que and 5-FU (13, 22). In those studies, 100 μM Que decreased VEGFR2 gene expression by approximately 80% and a combination of 0.5 mg/mL 5FU with 0.25 mg/mL bevacizumab reduced the VEGFR1 gene expression by approximately 25%, but in our study VEGFR2 gene expression was decreased by 50% with 130 μM Que, and 10% in combination of 130 μM Que plus 5 μM 5‑FU, which may be a result of the quality of purchased Que. Furthermore, to the best of our knowledge, their combination treatment and the evaluation of the enhancing effect of Que on the downregulation of mentioned receptors was not performed previously.
To evaluate the anti-angiogenic effects of Que, 5-FU, and their combinations, the CAM assay was conducted. Our results implied that branch points and total vessel network lengths significantly were decreased by applying Que and 5-FU alone compared to the untreated control group. The combination state caused a significant reduction in angiogenesis than 5-FU alone, which indicates the enhancement of the 5-FU anti-angiogenic effect via Que. The results of this part were consistent with a study that reported a significant decrease in the microvessel density following the co-treatment of 5-FU and the resveratrol in a tumor xenograft model (23). Moreover, in agreement with our results, in another study, it has been reported that the co-treatment of deoxypodophyllotoxin and 5-FU results in a significant reduction in the neovascularization (24). In that study, the combination of resveratrol (10 mg/kg) and 5-FU (10 mg/kg) decreased angiogenesis by 50%, but we reached to 60% decrease in angiogenesis with the combination of 130 μM Que plus 5 μM 5‑FU. The difference in the percentage of angiogenesis inhibition may be somewhat related to using the different methods.
5.1. Conclusion
In conclusion, the current study indicated that the combination of Que (130 μM) with 5FU (5 μM) improves the 5-FU anti-angiogenic effects compared to the application of 5-FU alone. Therefore, this combination could be suggested for the vivo studies in the future.