1. Background
Graft-versus-host disease (GVHD) is the Main Reason of Non-relapse mortality and morbidity after allogeneic hematopoietic stem cell transplantation (allo-HSCT) (1). According to properties of donors and recipients, human leukocyte antigen (HLA) compatibility, GVHD prophylaxis, and conditioning regimen, acute GVHD (aGVHD) approximately occurs in 30 - 50% of allo-HSCT patients (2, 3). In aGVHD, tissue damage is generally caused by T helper (TH)1 and TH17 cells and innate immune system cells such as natural killer (NK) cells (4). Damage-associated molecular patterns (DAMPs) that are released following conditioning regimens trigger innate immune responses, leading to the activation of adaptive immune responses that mostly manifest by inflammation in the gastrointestinal system, skin, and liver (4). Early detection of patients at risk for aGVHD may lead to closer monitoring, better prevention, and early treatment at disease onset (1). Therefore, utilizing specific biomarkers to predict GVHD is a necessary approach for its prophylaxis and treatment (1). Previous studies have introduced some plasma biomarkers such as interleukin (IL)-6, serum tumor necrosis factor receptor-1 (sTNFR1), sIL-2R, suppression of tumorigenicity 2 (ST2), and T cell immunoglobulin and mucin domain-3 (TIM-3) with high potential in predicting severe GVHD (5-8).
The TIM gene family was first described in 2001, and their role in regulating the immune system was discussed after that (9, 10). TIM family has 8 members (TIM 1 - 8) in mice and 3 members (TIM-1, TIM-3, TIM-4) in humans (11). TIM-3 was the first member to be extensively studied (9). TIM-3 is a co-inhibitory protein involved in inducing tolerance and inhibiting anti-tumor immunity (12). This molecule was originally thought to be a cell surface molecule that is selectively expressed on TH1 and CD8+ cytotoxic T cells (CTLs). Subsequent studies have shown that TIM-3 is also expressed on regulatory T (Treg) and TH17 cells as well as innate immune cells, including dendritic cells (DC), NK cells, and monocytes (13, 14). TIM-3 has several ligands including galectin-9 (Gal-9), carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), phosphatidylserine, and high mobility group box 1 (HMGB1), which have different binding sites on TIM-3 (15-19). Studies have shown that the interaction of TIM-3 with its main ligand, Gal-9, is involved in the regulation of immune responses, so-called immune exhaustion (12). TIM-3 inhibits the function of TH1 and TH17 cells (20). Based on the type of aGVHD, the inflamed tissues upregulate Gal-9. TIM-3/Gal-9 interaction is an essential regulatory process in transplantations (e.g. HSCT), chronic infections, tumors, autoimmune diseases, decreased T cell proliferation, cytotoxicity, and interferon (IFN)-γ secretion (21-23). In the models of GVHD mice, it was observed that the TIM-3 is overexpressed on activated TH1 effector cells (24). However, the exact role of TIM-3 in acute GVHD is obscure.
The TIM-3 molecule has both membrane and soluble forms. The increase in soluble (s)TIM-3 level has been shown in mouse models of aGVHD, supporting that sTIM-3 could be served as a biomarker for aGVHD (25).
2. Objectives
The aim of this study was to evaluate the association of plasma sTIM-3 level and aGVHD incidence in allo-HSCT patients.
3. Methods
3.1. Patients
This study was performed on 42 patients [22 (52.3%) males and 20 (47.7%) females] with a mean age of 32.50 ± 10.79 years and various hematological malignancies who underwent allo-HSCT from 2016 to 2019 at Hematopoietic Stem Cell Transplantation Center of Taleghani Hospital, Tehran, Iran. Besides, 20 healthy cases were included in the study as controls. The demographic and clinical data of patients, including disease, aGVHD incidence, conditioning regimen, GVHD prophylaxis, recipients ABO blood group, and donor-recipient relationship were collected from the clinical document. The study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (https://ethics.research.ac.ir/ProposalCertificateEn.php?id = 95125), and all participants signed the informed consent. All the procedures were performed as a part of the routine care.
The studied hematological disorders mainly included acute myeloid leukemia (AML), acute lymphoid leukemia (ALL), non-Hodgkin’s lymphoma (NHL), and Hodgkin’s disease (HD). All included patients received allogeneic stem cell transplants, which are fully matched in HLA-A, -B, -C, -DRB1, and -DQB1 loci. HLA typing was performed using a single specific primer-polymerase chain reaction (PCR-SSP) method as previously described (26). Patients were kept in isolated rooms with HEPA air filtration.
3.2. Conditioning Regimen
The myeloablative conditioning regimen received intravenously (IV) by all patients consisted of busulfan (BU; Otsuka, USA) 0.8 mg/kg every 6 hours for 4 days followed by either 2 days of cyclophosphamide (CY; Sandoz, Germany) 60 mg/kg/day or fludarabine (Flu; Genzyme, Germany) 30 mg/m2 once a day IV for 5 days. Reduced intensity conditioning regimen (RIC) utilized for patients with HD and NHL comprised of fludarabine 30 mg/m2 IV for 5 days, CCNU (Lomustine, Bristol Myers; USA) 100 mg/m2 P.O. for 2 days and Melphalan (Alkeran; Glaxosmith Klin (GSK) British) 40 mg/m2 IV for one day.
3.3. Peripheral Blood Stem Cell Isolation
Peripheral blood stem cells (PBSCs) were mobilized into the periphery after 4 days of subcutaneous administration of 5 or 10 µg/kg granulocyte-colony stimulating factor (G-CSF) (filgrastim, Amgen, USA) to the donors and harvested using apheresis Spectra Optia (Terumo BCT, Lakewood, CO) during 250 minutes depending on the volume and speed of donor blood PBSC flow. The CD3+ cells (FITC-conjugated human anti-CD3, Beckman Coulter, Miami, FL, US) and CD34+ cells (PE-conjugated human anti-CD34, EXBIO, Czech Republic) were counted by flow cytometry (Attune NxT, Invitrogen, USA). All patients received 5 × 108 and 2 - 4 × 106 mononuclear cells (MNC) and CD34+ cells/kg, respectively.
3.4. Neutropenia Phase Management
All patients received prophylactic antibiotic drugs during the neutropenic phase including oral acyclovir, fluconazole, and ciprofloxacin for viral, fungal, and bacterial infection, in the order given. Intravenous imipenem and vancomycin for febrile neutropenia and metronidazole for intestinal infection were administered. Moreover, patients received 5 µg/kg/day G-CSF IV from the day +1 (one day after transplantation) until the day that neutrophil counts increased to 1.5 × 109/L.
3.5. GVHD Prophylaxis and Diagnosis
All patients received 3 mg/kg/day of cyclosporine A (CsA; Sandoz, Germany) intravenously from day -2 to +5 (day of transplantation was considered as day 0) and 12.5 mg/kg/day P.O. until day +180 in combination with 10 mg/kg methotrexate (MTX; Sandoz, Germany) IV on day +1 followed by 6 mg/kg MTX IV on days +3, +6, and +11 as GVHD prophylaxis.
We used the NCC grading system (I-IV) for evaluating the incidence and the risk factor for diagnosis and grading GVHD in allo-HSCT patients (27). The standard clinical signs such as rash, diarrhea, and liver function abnormalities associated with biopsy and histopathological criteria in the involved organ were the primary manifestations for diagnosis of GVHD.
3.6. Collection and Processing of Blood Samples
Blood samples were collected at day +14 (before the onset of GVHD symptoms) from central venous access catheters in EDTA tubes. Plasma was isolated from blood samples using centrifugation at 1000g for 15 minutes, then cryopreserved at -80°C. Patients were classified as follows: (1) post allograft controls, with no evidence of acute GVHD at any time through day +100; (2) patients with grade 2 - 4 (severe) acute GVHD; and (3) grade 1 (mild) GVHD (28). Also, the blood samples were collected from 20 healthy individuals without HSCT as a control group to evaluate the plasma level of sTIM-3.
3.7. Measurement of Plasma sTIM-3
The measurement of sTIM-3 in the plasma was carried out by Quantikine enzyme-linked immunosorbent assay (ELISA) Human TIM-3 Immunoassay kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer. First, 100 µL of assay diluent was added to each well, followed by adding 50 µL of controls, standards, and samples and incubating for one hour at room temperature (RT) on a shaker set at 500 rpm. Afterward, the wells were washed 4 times with wash buffer, followed by adding 200 µL of horseradish peroxidase (HRP)-conjugated anti-human TIM-3 and incubating in the dark at RT for 30 minutes. The reaction was stopped by adding 50 µL of stop solution, and the optical density of each well was measured at 450 nm. The concentration of sTIM-3 in each sample was quantified based on the standard curve.
3.8. Statistical Analysis
The amount of sTIM-3 protein was provided as the mean and standard deviation (mean ± SD). The Shapiro-Wilk test was used to determine the normality of the data. Data analysis was evaluated using the student t-test and one-way ANOVA tests. P-values less than 0.05 were assumed significant. The statistical analyses were performed using SPSS software version 19 (IBM Corporation, Armonk, NY, USA).
4. Results
4.1. Patients Characteristics
A total of 42 patients who had received allo-HSCT in addition to 20 healthy cases as control were included in the study. The donor-recipient gender combinations were 12 (28.6%) male-male, 14 (33.3%) male-female, 6 (14.2%) female-female, and 10 (23.8%) were female-male. Acute myeloid leukemia (AML) was the most prevailing disease [n = 22 (52.4%)] followed by acute lymphocytic leukemia (ALL) [n = 10, (23.8%)], Hodgkin disease [n = 6, (14.2%)], and non-Hodgkin lymphoma [n = 4 (9.5%)]. Most of the patients, 32 (76.2%), received grafts from sibling donors (brother or sister) and 10 (23.8%) patients received grafts from non-sibling related donors. The results revealed that type O was the most frequent blood group (33.3%), closely followed by A (28.6%). According to the clinical manifestation, 18 (42.8%) patients developed aGVHD, while 24 (57.2%) patients did not have GVHD manifestation. Among 18 patients with aGVHD symptoms, 10 (23.8%) had grade 2 - 4 GVHD; and 8 (19%) experienced grade 1 GVHD (Table 1).
| Characteristics | Values a |
|---|---|
| Recipient age | 32.50 ± 10.79 |
| Donor age | 33.82 ± 11.22 |
| Diagnosed disease | |
| NHL | 4 (9.5) |
| HD | 6 (14.3) |
| AML | 22 (52.4) |
| ALL | 10 (23.8) |
| Donor-recipient relationship | |
| Sibling | 32 (76.2) |
| Related | 10 (23.8) |
| Donor-recipient genders | |
| Male-male | 12 (28.6) |
| Male-female | 14 (33.3) |
| Female-female | 6 (14.3) |
| Female-male | 10 (23.8) |
| Conditioning regimen | |
| Bu/Cy | 22 (52.4) |
| Bu/Fu | 10 (23.8) |
| Bu/Fu/ATG | 4 (9.5) |
| RIC | 6 (14.3) |
| GVHD prophylaxis regimen | |
| CSA+MTX | 42 (100) |
| ABO blood group | |
| A | 12 (28.6) |
| B | 10 (23.8) |
| AB | 6 (14.3) |
| O | 14 (33.3) |
| aGVHD incidence | |
| aGVHD | 18 (42.8) |
| Grade 2 - 4 (severe) | 10 (23.8) |
| Grade 1 (mild) | 8 (19) |
| No aGVHD | 24 (57.2) |
Descriptive and Clinical Data of Patients
4.2. Plasma Level of sTIM-3
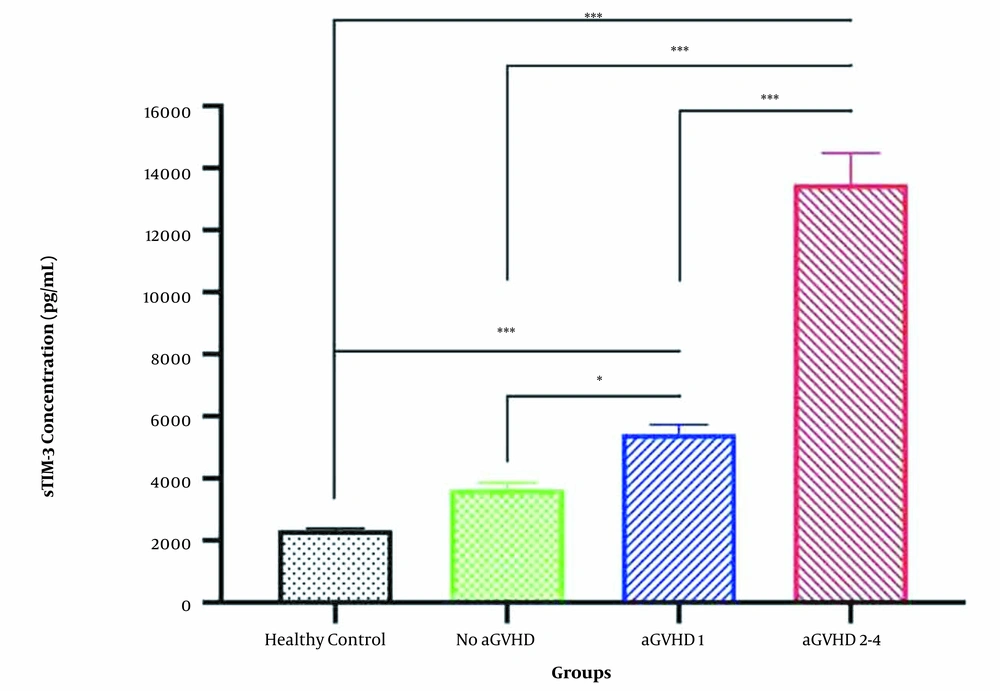
Blood samples were collected on day +14 from allo-HSCT patients and healthy controls and plasma levels of sTIM-3 were measured. According to Table 2 and Figure 1, sTIM-3 levels at day +14 were significantly higher in patients who developed aGVHD (whether mild or severe) compared to the healthy control individuals who did not undergo HSCT (P-value < 0.001). No significant difference was seen between the sTIM-3 levels in the healthy group (mean level of plasma sTIM-3 = 2344 pg/mL) and allo-HSCT patients who did not develop aGVHD (mean = 3665 pg/mL). The plasma level of sTIM-3 in the allo-HSCT patients who did not develop aGVHD was significantly lower than those who manifested grade 1 aGVHD (P-value = 0.015) and grade 2 - 4 aGVHD (P-value < 0.001). Among the aGVHD patients, the sTIM-3 levels in those with grade 2 - 4 aGVHD were significantly higher (approximately 2.5 times) than those with grade 1 aGVHD (P-value < 0.001) (Figure 1). The mean plasma level of sTIM-3 in grade 1 aGVHD patients was 5432 pg/mL, and the mean plasma sTIM-3 level in grade 2-4 aGVHD patients was 13486 pg/mL (Table 2).
| Groups | Patients (N) | TIM-3 Concentration (Pg/mL) |
|---|---|---|
| GVHD | ||
| Total | 18 | |
| Grade 2 - 4 | 10 | 13486 ± 577.4 |
| Grade 1 | 8 | 5432 ± 173.2 |
| No GVHD | 24 | 3665 ± 115.5 |
| Control | 20 | 2344 ± 28.9 |
Plasma Level of TIM-3 in Groups
5. Discussion
Despite all benefits of allo-HSCT for patients with cancer, this treatment is confined by high morbidity and mortality arising from GVHD (29, 30). The expression of TIM-3 on immune cells is highly variable and is dependent on the cell subtype and immune status (20). It has been reported that the TIM-3 expression on normal human blood T cells is low or undetectable (12). Conversely, TIM-3 is constitutively expressed in the innate immune system cells such as NK cells, monocytes, and DC (18, 20). TIM-3 signaling in T cells induces apoptosis and/or blocks T cell effector functions responsible for acute inflammation and GVHD onset (8, 24). Previous studies have shown that the interaction of TIM-3 and Gal-9 plays a critical role in the regulation of the immune response in autoimmunities, chronic infections, tumors, and transplantations including GVHD (12, 31-33). TIM-3 is known to be an immune inhibitory molecule (20). So, it might be presumed that its overexpression is indicative of anti-inflammatory states. However, evidence showed that TIM-3 upregulation might result from inflammatory responses (14). First, the immune cells are activated and respond to antigens; then, they upregulate the inhibitory molecules to restrain their functions (14, 34). Therefore, the inhibitory checkpoints could indicate the pre-existing (or maybe chronic) inflammatory condition (34). Human studies on transplant recipients suggested that TIM-3 can be considered a marker of TH1 cell activity and transplant rejection (35). Studies in the allograft transplants have shown that patients who rejected the transplant had a significant increase in the mRNA level of the TIM-3, and there is a strong correlation between IFN-γ and TIM-3 levels within the tissue (36). Overexpression of TIM-3 was shown in mouse models of aGVHD, and the inhibitory function resulting from the interaction of TIM-3/Gal-9 might play a role in the pathogenesis of aGVHD (25). Therefore, the plasma level of sTIM-3 could be one of the predictive or prognostic factors for GVHD. The study of plasma samples of patients with GVHD using mass spectrometry by a group of researchers in 2013 led to the identification of the soluble form of the molecule TIM-3 (31). They believe that sTIM-3 is caused by releasing the second extracellular molecule of TIM-3 into the bloodstream (31), but its exact mechanism is currently unknown. The level of sTIM-3 in the GVHD patients increased before the onset of GVHD clinical symptoms, and a significant relationship between its levels and the severity of GI-GVHD was also identified (25, 31). Therefore, it has been suggested that increased plasma levels of sTIM-3 may have roles in the pathogenesis by inhibiting the binding of TIM-3 to its ligand and interfering with the regulatory activity of TIM-3 molecules on the surface of T cells (31). The sTIM-3 in the plasma could be passively released from apoptotic cells of GVHD patients (8, 37). In our study, the higher plasma levels of sTIM-3 in severe GVHD patients confirm the association of sTIM-3 with aGVHD. This is consistent with the report of Hansen et al. that revealed increasing in sTIM-3 plasma level predict GVHD with grade 2 - 4 (31). We also observed the plasma level of sTIM-3 in grade 2 - 4 aGVHD patients was increased compared to grade 1 aGVHD patients. Decreased plasma concentrations of sTIM-3 may serve as a therapeutic target for limiting GVHD activity and promoting tolerance. sTIM-3 measurement was introduced as a useful biomarker for predicting aGVHD grades 3 - 4, rather than grades 1 - 2 (38).
5.1. Conclusions
Our results showed that the plasma level of sTIM-3 in grade 2 - 4 aGVHD was higher compared to grade 1 aGVHD and control group. Our results are in agreement with previous studies that reported that the sTIM-3 plasma level in aGVHD was increased, and this could be a predictive biomarker of GVHD in allo-HSCT patients (8, 37). Understanding the TIM-3-mediated immune regulation can provide insight for new approaches to enhance the prevention of the alloimmune response. In conclusion, we have identified a high plasma level of sTIM-3 as a predictive biomarker of acute GVHD, especially grades 2 - 4, among allo-HSCT patients.