1. Background
Rectal cancer comprises approximately one-third of colorectal cancer, making it the seventh most prevalent cancer globally (1). The incidence of colorectal cancer is growing in Iran, which might be due to the changes in dietary patterns (2). Recently, mortality of colorectal cancer has been decreased due to screening programs, early detection, as well as improvement in treatment options.
Treatment of locally advanced rectal cancer usually initiates with neoadjuvant chemoradiation. The use of neoadjuvant chemoradiation has led to a decrease in local recurrence and an increase in pathologic complete response (3, 4). Loco-regional recurrence of rectal cancer is difficult to treat and is associated with a poor prognosis (5).
2. Objectives
The aim of this study was to investigate the feasibility and efficacy of high dose rate (HDR) brachytherapy (BRT) boost in rectal cancer.
3. Methods
This retrospective study was performed to investigate the effect of HDR brachytherapy boost on pathologic response and local control of non-metastatic rectal cancer patients. This trial was conducted in the Radiation Oncology Department of Mahdie Radiotherapy Center, Hamadan University of Medical Sciences, Hamadan, Iran. Patients information was coded and confidential.
Medical records of patients with rectal cancer, who were treated in the radiation oncology ward, were assessed. Patients were required to meet the following inclusion criteria: age over 18 years, histologically confirmed adenocarcinoma of the rectum, local staging by MRI and/or EUS demonstrating a T2-T4 and/or node-positive tumor, and ECOG performance status of 0 or 1. Exclusion criteria were tumors lactated more than 12 cm from the anal verge, metastatic disease at presentation, positive inguinal or iliac lymph nodes on MRI, PET, or EUS, concurrent malignancy, bulky tumors that would not allow application of the endorectal probe, and previous pelvic irradiation. Considering the inclusion and exclusion criteria, 15 patients who were treated with HDR brachytherapy boost were considered as the intervention group. Fifteen patients who were clinically matched (age, sex, stage, distance of tumor from anal verge) were selected as the control group. The rate of PCR, downstaging (T staging), and frequency of side effects were compared between the two groups of the study.
All patients were preoperatively staged by endorectal ultrasound and/or pelvic MRI. Computed tomography of the chest, abdomen, and pelvis was performed to rule out distant metastasis. All patients received neoadjuvant chemoradiation (50.4Gy in 28 fractions), using 3D-conformal radiotherapy with concurrent capecitabine (825 mg/m2 twice a day for 5 days per week). The target volume included the tumor, perirectal fat tissue, and lymph nodes (pre-sacral, internal iliac, and obturator). HDR brachytherapy was done with the endorectal cylinder with an iridium 192 source within 7 to 10 days after the completion of external beam radiotherapy. BRT was given 3Gy/Fr for 3 sessions. The interval between sessions was 5 to 7 days. A 3-Gy dose was prescribed at a 5-mm distance from the mucosal surface. All patients underwent total mesorectal excision (TME) in a referral center within 4 to 8 weeks after completion of neoadjuvant treatment. Pathological report of the surgical specimen was done by two pathologists with more than 10 years of experience. Also, all the patients received 6 months of perioperative chemotherapy with intravenous oxaliplatin 130 mg/m2 (day 1) followed by oral capecitabine 1000 mg/m2 twice a day (day 1 - 14). Two patients in the control group also received two cycles of induction chemotherapy with the same regimen. After completion of treatment, patients were followed every 3 months with CEA and physical exam.
The statistical analysis was done by SPSS version 24. Pearson's chi-squared test was conducted to compare the binominal parameters in the two groups. The quantitative variables were compared between the control and intervention groups, using a t-test. P-value < 0.05 was the critical criterion for statistical significance.
4. Results
The present study was performed on 30 patients referred to the radiotherapy center from May 2020 to May 2021. The mean age of patients was 57.97 ± 9.11 years and 18 patients (60%) were male. The patients were divided into control (n = 15) and intervention (n = 15) groups. Demographic and baseline characteristics of the patients were assessed. The results showed that T3 and N1 rectal cancer had the highest frequency (76.7% vs. 43.3%, respectively). Concurrent Capecitabine was the most common type of chemotherapy among the patients (93.3%). There was no significant difference between the two groups of control and intervention based on demographic and baseline characteristics (P > 0.05). Table 1 shows the demographic and baseline characteristics of the patients participating in the control and intervention groups.
| Variables | Total | Group | P-Value | |
|---|---|---|---|---|
| Control (n = 15) | Intervention (n = 15) | |||
| Gender | 0.456 | |||
| Female | 12 (40.0) | 7 (46.7) | 5 (33.3) | |
| Male | 18 (60.0) | 8 (53.3) | 10 (66.7) | |
| Age (y) | 57.97 ± 9.11 | 56.33 ± 7.29 | 59.60 ± 10.62 | 0.394 |
| T stage | 0.686 | |||
| 2 | 4 (13.3) | 1 (6.7) | 3 (20.0) | |
| 3 | 23 (76.7) | 12 (80.0) | 11 (73.3) | |
| 4 | 3 (10.0) | 2 (13.3) | 1 (6.7) | |
| N stage | 1.000 | |||
| 0 | 6 (20.0) | 3 (20.0) | 3 (20.0) | |
| 1 | 13 (43.3) | 6 (40.0) | 7 (46.7) | |
| 2 | 11 (36.7) | 6 (40.0) | 5 (33.3) | |
| Chemotherapy | 0.483 | |||
| Concurrent | 28 (93.3) | 13 (86.7) | 15 (100.0) | |
| Induction | 2 (6.7) | 2 (13.3) | 0 (0.0) | |
| AV distant | 6.13 ± 3.02 | 5.66 ± 3.19 | 6.60 ± 2.86 | 0.368 |
| CEA b | 8.02 ± 11.51 | 5.42 ± 3.38 | 10.63 ± 15.77 | 0.589 |
a Values are expressed as mean ± SD or No. (%).
b CEA measured at presentation before initiating any treatment.
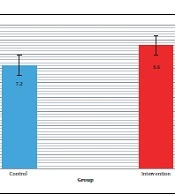
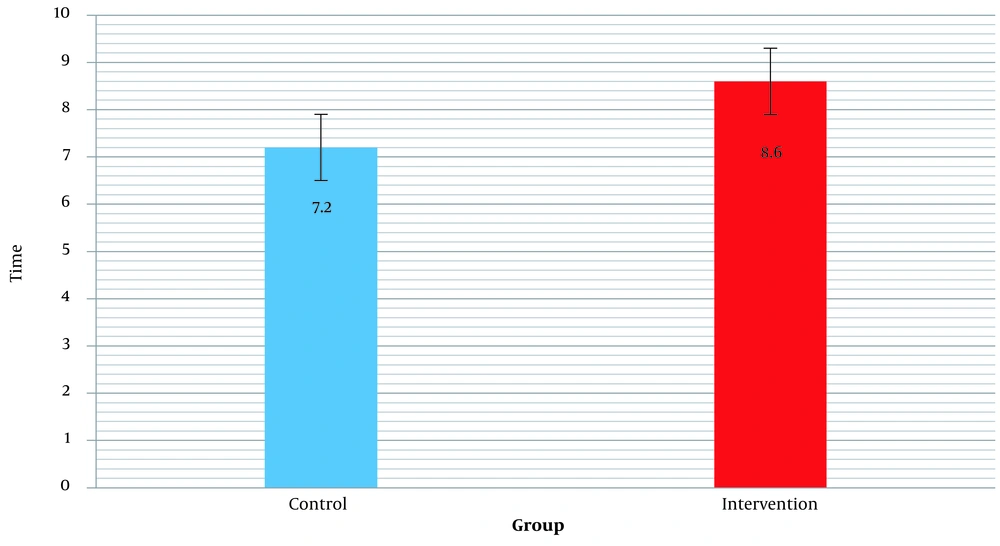
The mean "Follow-up time" from the last day of radiation therapy to the last patient’s visit was evaluated in the control and intervention groups, which showed that there was no significant difference between the control and intervention groups (P = 0.231). Table 2 shows the mean and standard deviation of the mean "Follow-up time" for the two groups of control and intervention in patients. Figure 1 shows the bar chart of the mean "Follow-up time" in the control and intervention groups.
| Variables | Mean ± SD | P-Value |
|---|---|---|
| Follow-up time (mo) | 0.231 | |
| Control | 7.20 ± 2.85 | |
| Intervention | 8.60 ± 3.83 |
Examination for "T downstaging" after surgery in the patients showed that there was no significant difference between the control and intervention groups after surgery (66.7% and 80% respectively, P: 0.40). Table 3 shows "T downstaging" after surgery in the control and intervention groups.
| Variables | Downstaging of T | P-Value | |
|---|---|---|---|
| Control (n = 15) | Intervention (n = 15) | ||
| T downstaging | 10 (66.7) | 12 (80.0) | 0.40 |
| Pathologic complete response | 2 (13.3) | 2 (13.3) | > 0.99 |
a Values are expressed as No. (%).
"Pathologic complete response" was investigated and the results showed that there was no significant difference in the control and intervention groups (13.3% in both groups, P > 0.99) (Table 3).
During the follow-up period of 3 months, no significant difference was seen between the two groups in terms of complications (Table 4).
| Side Effect | Groups, No. (%) | |
|---|---|---|
| Control (n = 15) | Intervention (n = 15) | |
| Infection (wound) | 0 (0.0) | 1 (6.6) |
| Anastomotic leakage | 0 (0.0) | 0 (0.0) |
| Fistula | 0 (0.0) | 0 (0.0) |
| Urinary problems | 2 (13.3) | 1 (6.6) |
| Stenosis | 0 (0.0) | 0 (0.0) |
5. Discussion
Rectal cancer is one of the most known cancers worldwide, especially in Western societies (6). In Iran, it is the third most common cancer among men and the fourth most common cancer among women, whose prevalence has been growing in recent years (7). The treatment of rectal cancer over the past two decades has changed considerably. Radiotherapy whether as a short course or long course has resulted in improved prognosis and decreased local relapse (8). The standard surgical treatment in these patients is abdominopelvic resection (APR) and low anterior resection (LAR) alongside total mesorectal excision (TME) (6). Patients with pathologic complete response (PCR) have had higher survival compared to those with the low response (9). The factors affecting PCR include a gap between radiotherapy and surgery above 8 weeks, tumor size smaller than 5 cm, high T, mucinous type, and CEA level (9-11).
Different studies have investigated the effect of adding brachytherapy to rectal cancer treatment. In the study by Vuong et al., in assessing 49 patients suffering from rectal cancer undergoing brachytherapy with a 26 Gy dose in 4 fractions followed by surgery, brachytherapy played a significant role in downstaging and preserving the sphincter in the postoperative pathology (12).
The study of Rijkmans et al. investigated the factors affecting response to HDR brachytherapy in rectal cancer. In that study, tumor size smaller than 2 cm at the baseline and response to EBRT were among the most important factors affecting clinical complete response (13).
In a study by Appelt et al. in investigating 221 patients suffering from rectal cancer undergoing long course neoadjuvant chemoradiation treatment with or without brachytherapy boost of 10 Gy in two fractions, the brachytherapy boost was associated with increased tumor regression, but OS and PFS did not differ significantly (14).
The systematic review by Buckley et al., covering 12 studies on combinational therapy of HDR-BT plus EBRT, resulted in 18 to 31% PCR, and insole HDR-BT led to 10 to 27%. That study concluded that HDR-BT, either alone or combined with EBRT, caused improved PCR. However, the studies had significant variations in patient selection, brachytherapy technique, and surgical treatment (15).
Brachytherapy boost may be used as an alternative treatment to surgery in case of the development of CCR. In the study by Sun Myint et al. on 83 patients suffering from rectal cancer who had residual less than 3 cm following EBRT, the patients underwent brachytherapy. CCR was around 63%. These patients did not undergo surgery, and in the 2.5-year follow-up, 83% of the patients were cancer-free, and the rate of local relapse was 13% (16).
Our study has retrospectively investigated the rate of PCR in case of adding brachytherapy boost to standard treatment. However, there was no significant difference between the two groups in terms of PCR. In a randomized trial by Jakobsen et al. that compared the efficacy of brachytherapy boost (10 Gy/ 2 fractions) to the standard regimen, the rate of PCR was 18% in both control and intervention groups, but the rate of major response was significantly higher than in BRT boost group (14). In our study, although the rate of downstaging using T staging was 14% higher in the BRT boost group, the difference was not statistically significant. This result might be due to the small sample size, the difference in response assessment, and the differences in the applied technique and dose.
The results of this study should be interpreted considering its limitation. The first limitation is the retrospective nature of the study. The second is the small sample size and the third one is the method of response assessment. Unfortunately, tumor regression grade was not routinely reported at the time; so, T staging was used in this study. It has to be mentioned that two patients in the control group received two cycles of induction chemotherapy outside of the protocol, which might have affected the results.
5.1. Conclusions
Although a BRT boost is feasible and might improve downstaging in rectal cancer, it could not increase the rate of PCR.