1. Background
One of the common antioxidants is Vitamin E (Vit E), which acts against reactive oxygen species (ROS) on the cell membrane and microenvironments (1). Many reports have proved that Vit E can protect against the toxicity effects of doxorubicin (DOX) through the antioxidant ability (2). Lipid peroxidation is a harmful factor that could be decreased by the level of glutathione related to Vit E level of the small intestine (3). Numerous studies have shown that Vit E having antioxidant activities protects against Carbon tetra chloride (CCl4)-induced hepatotoxicity by inhibiting lipid peroxidation and enhancing antioxidant enzyme activity (4). The amount of Vit E activates the improvement of the antioxidant enzymes and reduction of the oxidative stress in the livers, which are exposed to Cadmium (5, 6). A combination of soy isoflavone as an adjunct to docetaxel chemotherapy can be effective in improving diet consumption in breast cancer (7).
Omega-3 (Ω-3) fatty acids mainly exist in fish oils and can reduce the effects of ROS. It has an anti-inflammatory effect that helps to reduce myocardial inflammation, arteritis, and thrombosis development (8). The development of atherosclerosis in pigs with hyperlipidemia has been reduced through omega-3 supplementation (9). Omega-3 is effective in controlling sepsis, endotoxemia, and inflammation (10). Omega-3 also could induce death or slow the growth of cancer cells in culture media and in vivo experiments. In one study, a 3% fish oil concentrate was supplemented synchronously with DOX for caring for cancer xenografts. Interestingly, this omega-3 significantly increased oxidative stress in tumors, not as similar as in the liver. It also significantly prevented the growth rate of cancer (11).
The medication DOX prefers treating effects on many neoplasms, such as hematological ones. However, anticancer drugs could seriously disturb many organs, such as the liver, kidneys, and various vessels (12). The liver is the sensitive point weak in DOX damage because of high metabolic capacity. DOX could generate and start reactive molecules, including oxygen radicals. DOX could inactivate glutathione peroxidase (GPx) and Superoxide dismutase (SOD). Then, the quantity of ROS presentation and antioxidant ratio could lead to more tissue destruction. It is confirmed that the DOX-induced ROS and its metabolites begin necrosis and apoptosis using caspase three and receptors in the liver (13). Detoxification of many drugs and toxins is ongoing by the liver. The lesions of the liver are in a varying range of tissue damage. These are categorized as hepatocellular necrosis, apoptosis, vacuolar degeneration, portal, and parenchymal inflammation. Hepatotoxic drugs induce changes in the cytoplasmic, membranous, and chromatin of the cells. The result of the effects of the toxic drugs is acute hepatic failure (14). The clinical approach for using toxic anticancer therapy is not surprising and may be harmful to the liver. Hepatic failure or lesions due to toxic agents can be so severe that be lead to death. We try to evaluate the protective ability of some antioxidants against hepatotoxic drugs in mice.
Superoxide dismutase (SOD) is a metalloprotein containing both copper and zinc ions in each subunit. It is a well-known antioxidant that obliterates ROS production (12). One of the most important organelles that have an important role in ROS production is peroxisomes. They can increase β-oxidation, which leads to a considerable amount of ROS. Notably, if the catalase goes depleted in the peroxisomes, the induction of pexophagy and ROS production could occur in cells. In one study, mice with knockout catalase genes can increase ROS induction in the liver, which can increase the pexophagy of peroxisomes. Pro-inflammatory cytokines could be increased that related to ROS generation (15). Cisplatin and Doxorubicin can produce many ROS in parenchymal tissues such as the liver that induce liver toxicity. They, then, elevate related enzymes including alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyltransferase (GGT), alkaline phosphatase (ALP), and total bilirubin. Some investigators proved that dietary omega-3 and vitamin E improves immunodeficiency and increases the survival range for patients with cancers. The pathological evaluation can determine the toxic injuries (16).
2. Methods
2.1. Nutrients Preparation
Vit E (Vit E from Health Aid, U.K) Ω-3 Fatty Acid (Ω-3 FA from Formulated Sciences, Canada), and Doxorubicin (DOX, Cell Pharma, 50 mg per 25 ml) were afforded.
2.2. Animal
This investigation was achieved on thirty 25 g balb/c mice. In the beginning, all mice were owned in cages for fourteen days and fed with a similar diet. The examined animals were separated into 5 experimental groups of 6 mice each. The groups were as follows: Control; Animals fed only normal saline (1 mL/kg) for 10 days; DOX: Doxorubicin was injected at a dose of 15 mg/kg intraperitoneal (17). Vit E; mice were daily administered with Vitamin E, 400 IU/kg, (18) for 10 days followed by 1 dose DOX injection at 15 mg/kg intraperitoneal. Ω-3: Mice were daily administered with Omega-3, 400 mg/kg, (19), for 10 days followed by 1 dose DOX injection at 15 mg/kg intraperitoneal. Both Vit E- Ω-3: Mice were daily administered with both 400 IU/kg Vit E and 400 mg/kg Ω-3, for 10 days followed by 1 dose DOX injection at 15 mg/kg intraperitoneal. Our curing and treating behavior of animals was according to Legislation for the protection of animals used for scientific purposes.
2.3. Biochemical Blood Parameters
Blood samples were taken from the cervical vein, and all animals were euthanized ethically. The level of seromic enzymes, including ALT, AST, GGT, ALP, and total bilirubin, were analyzed using an automatic biochemical analyzer on day 20. (Accent 200, China).
2.4. Liver Tissue Antioxidants
(1) We determined the catalase activity by the colorimetric method (20). We produced the homogenate of liver tissue and incubated it with Hydrogen peroxide. We stopped the enzyme reactions by the addition of ammonium molybdate. We reported catalase activity as U/ml.
(2) We used a spectrophotometric assay by Zellbio kit to analyze Glutathione peroxidase (GPx) activity. The measurement of this parameter was based on the rate of absorption in the ELISA Microplate Readers (21).
2.5. Histopathological Sample Preparation and Evaluation
The liver samples were poured in 10% formalin buffer for histological evaluation and immunohistochemistry. The hepatic tissues grade of lesions were achieved by 0 = absent; 1 = low or weak; 2 = mild; 3 = moderate; and 4 = high or frequent, and the total score was the basis of judgment system (22).
2.6. Immunohistochemical Sample Preparation
Antibodies against TNFα were operated on to demonstrate the inflammation. Avidin and biotin peroxidase were utilized to notice the TNFα. The 6-µm sections were exposed to TNFα monoclonal antibody (dilution 1:100; DAKO). Five high-power fields were observed at random.
2.7. Statistical Analyses
The mean ± SEM of the data and one-way ANOVA were analyzed. Only P < 0.05 was noticed as significant. GraphPad Prism was the software used for analyzing and drawing graphs.
3. Results
3.1. Toxicity
The mice in Vit E or Ω-3 groups lived with good body states and weight. DOX created many side outcomes, including hair loss, poor body condition, lower movement, and liability.
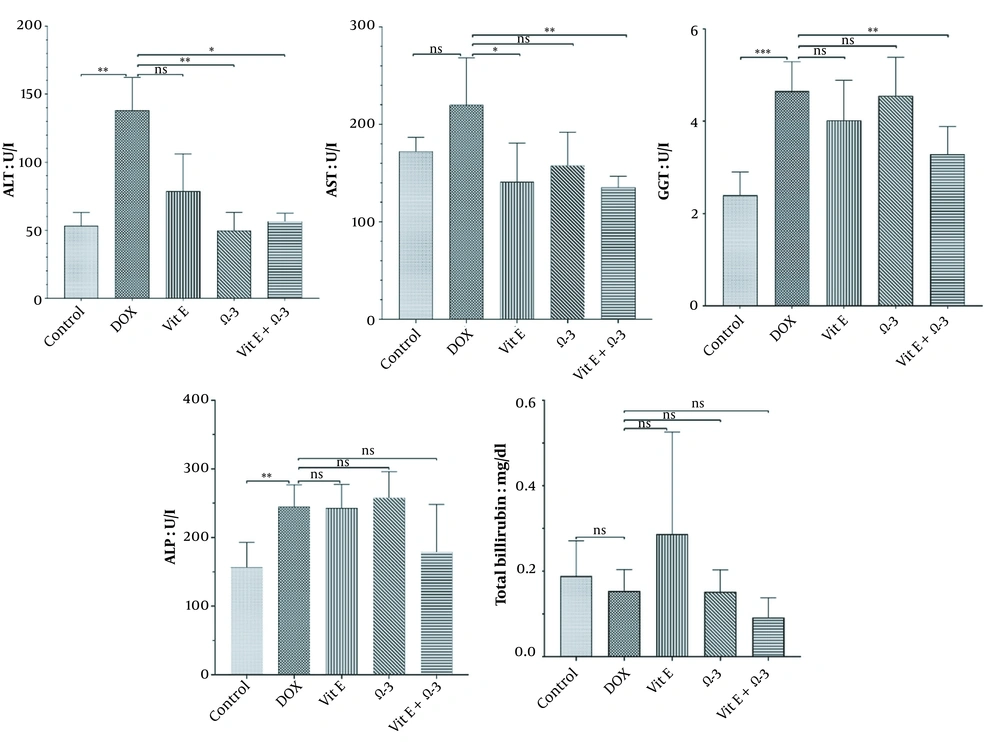
3.2. Biochemical Parameters
The ALT, AST, GGT, ALP levels, and total bilirubin were analyzed. The findings showed a non-significance relation (P < 0.05) between the DOX group and the others (Figure 1).
3.3. Liver Tissue Antioxidants
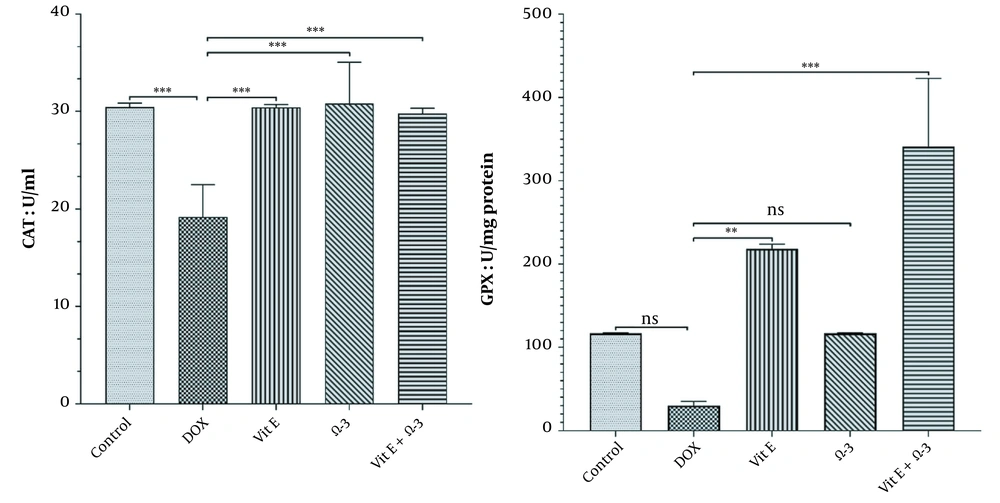
3.3.1. Catalase Activity
There were significant differences in the amount of CAT in the liver parenchyma between the DOX group and the others (***) similarly (Graph 2, P < 0.05).
3.3.2. Glutathione Peroxidase Activity
The amount of Gpx enzyme in the Vit E group was increased more significantly (**) than in the doxorubicin group. This improvement effect was better in the Vit E + Ω-3 group (***) than in the others (Figure 2, P < 0.05).
3.4. Histopathological Structure of the Life
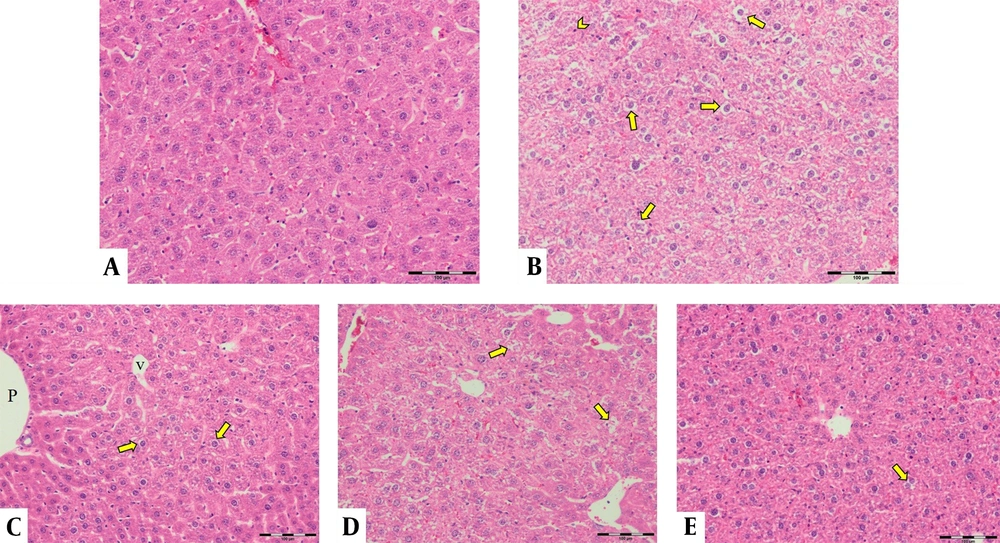
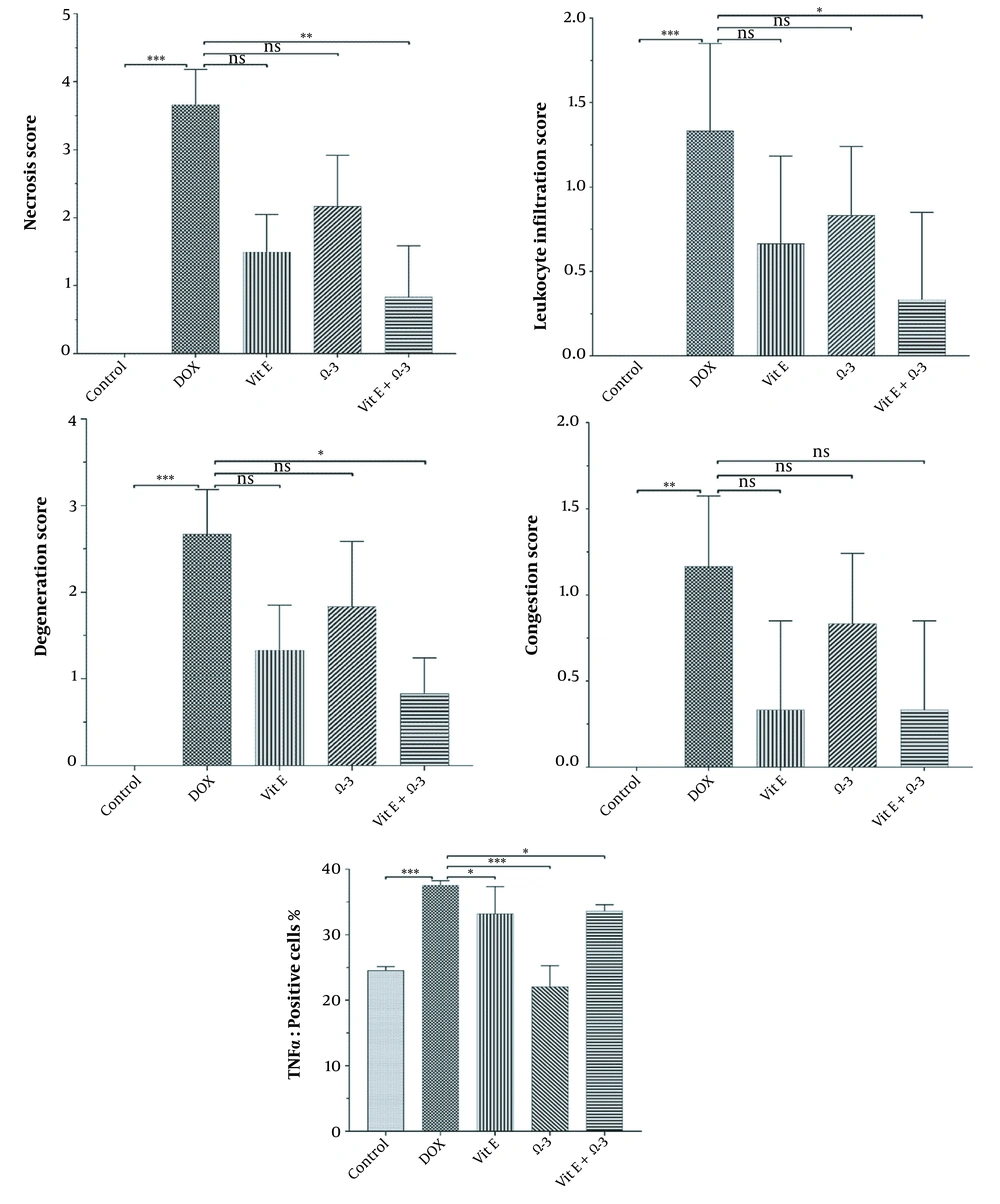
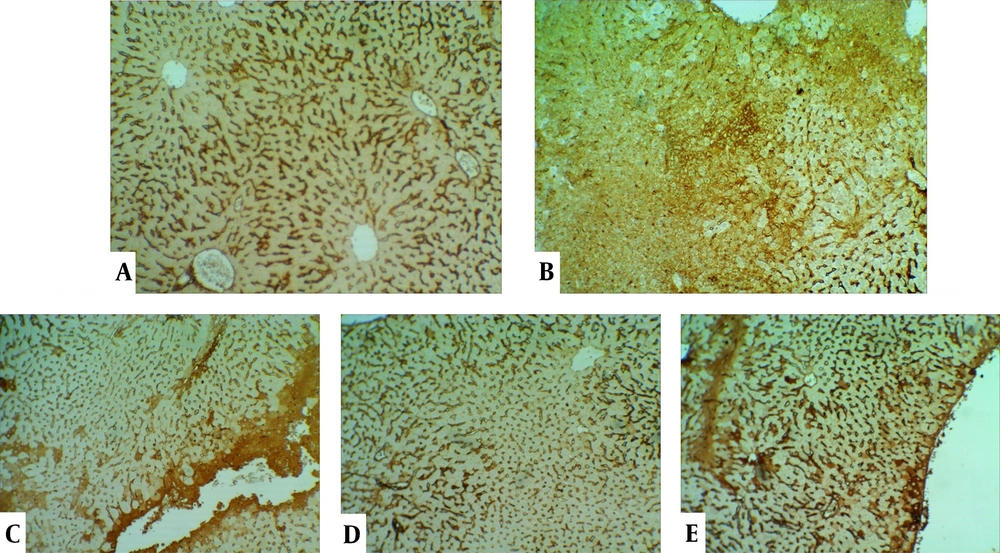
The control group's liver showed a normal structure of hepatocytes, central veins, and portal areas (Figure 3A). The DOX group showed severe lesions, including necrosis, inflammation, and vacuolar degeneration (Figure 3B). Nuclear condense, and cytoplasm loss was prominent in the necrotic area. The DOX group was significantly worse than other study groups. The centrilobular degeneration of the livers in the Vit E group showed better improvement than Ω-3 because of the diffuse appearance of the latter. The Vit E and Ω-3 administration had the most ameliorating effects (Figure 3C - E, and Figure 4, P < 0.05).
Histopathological evaluation of the livers. (A) control group not exposed to doxorubicin. (B) DOX group we treated with doxorubicin. (C) Vit E, (D) Ω-3, and (E) Vit E+ Ω-3 compared to DOX group (P < 0.05); (HE×200). Yellow arrow: Vacuolar degeneration; Chevron: Necrotic hepatocyte; P: Portal vein; V: Central vein.
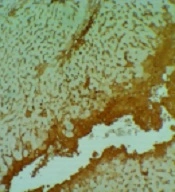
There are many significant differences between all groups in necrosis, inflammation, and degeneration, especially in the control versus DOX groups. No differences were among the groups in the congestion appearance except for the DOX group (P < 0.05). In addition, there are many significant differences between DOX versus other groups in the amount of TNFα in the liver (P < 0.05).
3.5. Immunohistochemistry Analysis of TNFα in the Liver
Expansive TNFα expression of the DOX group demonstrated an inflammatory process and toxic effects. Vit E groups had more potential for reducing lesions than the Ω-3 group. The Vit E-Ω-3 groups significantly showed the best TNFα expression decline (Figure 5A - E).
4. Discussion
DOX is a potent free radical inducer in many organs. The body's antioxidants cannot eradicate radicals separately (23). When the mitochondrial enzymes metabolize the DOX, many hepatocytes are destroyed (24). Mitochondrial oxidation-reduction and ROS could lead to the degradation of DNA, Apoptosis, and Necrosis in the liver. (25). The effects of Vit E, Ω-3, and both of them were studied as anti-inflammatory aspects. Their protection abilities were assessed against hepatic damage. Vit E is an antiradical agency that modifies the liver's results of free oxygenous radicals (26). Vit E can decrease ischemia-reperfusion tissue damage (27). Some investigators have shown that small vessels' killed endothelium will enhance after the Vit E regime (28, 29). Vit E regime therapy controls neoplasms growth and protects against chemotherapic side effects (30). The Ω-3 can enhance the liver lesions that drugs and pathogens generate ROS (31-33). The Ω-3 displayed a significant defensive influence against the effects of CCL4 lesions. The Ω-3 showed a significant reduction of AST, ALT, ALP, and total bilirubin in the CCL4-related animals. (33, 34). Co-administration of Vit E or Ω-3 with DOX could prevent increased serum ALT and AST levels (12, 18). The level of ALP is a marker to determine cholestasis in mice (35). Co-administration of Vit E and Ω-3 could diminish the level of ALT, AST, GGT, ALP, and total bilirubin, more imaginable than each of them alone. DOX did not induce cholestasis because of the low level of the serum ALP. The Vit E regime may facilitate hepatic lesions. Administration of Vit E can protect against the vascular degeneration which DOX produces. The Ω-3 could enhance the liver lesions of ROS (33, 34). The Ω-3 400 mg/kg histologic appearance may reduce lipid oxidation in the liver. NADPH enzyme of the mitochondria produces ROS with Doxorubicin-administration (36). NADPH is a reducing factor that the enzyme named nitric oxide synthase (NOS) could utilize to produce NO from L-arginine. L-arginine deficiency can lead to superoxide construction (37). Vit E and Ω-3 are detoxifiers and antioxidants that inhibit ROS synthesis (18, 32, 34). According to this study, using Vit E and Ω-3 significantly heals hepatic damage. Microscopically, treatment with DOX could produce leukocyte infiltration, congestion, and vacuolar degeneration (23). Ω-3 treatment can improve the antitumor ability of DOX (38). In addition to the pathologic outcomes of DOX toxicity, many growth factors that may induce bile duct hyperplasia and inflammation are synthesized (39).
The degree of lobular necrosis in the DOX group was moderate despite the severe degree of sinusoidal engorgement, vacuolar degeneration, and inflammation. The degree of all lesions in the treated groups was mild. The rate of necrosis in the doxorubicin group was much higher than in the other groups. Necrosis in the Vit E group was less than Ω-3. However, combined administration of both could be more effective than Vit E alone. The rate of leukocyte infiltration in the doxorubicin group was much higher than in the control group. The Ω-3 group has no more infiltrated leukocytes compared to the vitamin E group. However, combined administration of both could be more effective in reducing leukocyte infiltration than either alone. Hepatocytes in the doxorubicin group underwent vacuolar degeneration. In the control and both Vit E- Ω-3 together groups, the degree of degeneration was significantly lower. This badge means that Vit E or Ω-3 alone cannot inhibit hepatocyte degeneration compared to using both of them. There was no significant difference in the degree of sinusoids and central vein hyperemia in all groups (Figure 4). Cytokines are small and membrane-bound immunomodulating proteins that aid cell-to-cell communication in immune responses and stimulate the movement of cells toward sites of inflammation, infection, and trauma (39). TNF-α is an initiator cytokine that could start other cytokine cascades, including IL-1β synthesis, which can overstate the inflammation (40). The most way that DOX produces injuries to many organs is because of the induction of releasing inflammatory cytokines after necrosis and degeneration of the tissues. Both Vit E and Ω-3 can reduce the serum levels of TNF- α, IL-6, IL-1β, and IL-17 in mice. They can increase the levels of IL-10 (10, 33, 41, 42). When toxins create necrosis or other injuries, an inflammasome named NOD-like receptor protein three could exaggerate the inflammation. After that, the activated inflammasome could induce more action in cancer and diabetes. Vit E diminishes inflammation by inhibiting the synthesis of a kind of Nod-like receptor-mounted NLRP3 (41). In this study, the DOX group's TNF-α expression in the liver means more inflammation (43).
We found that the administration of Ω-3 or Vit E separately in the other experimental group decreased the level of TNF-α in the liver. Using synchronous of both could reduce lesions more than individual supplementation only. The reduction of the TNFα, the anti-inflammatory effects, and the antioxidant and anti-inflammatory effects we describe in this experiment for the Ω-3 group have been observed in the Vit E group better than in the Ω-3 group. In addition, it was more than improved by their co-administration of them. On the other hand, the administration of the Ω-3 alone could not give us the same optimal effects.
The combination of vitamin E and Ω-3 may reduce the cytotoxicity induced by doxorubicin (44). These facts require further studies. This article tried to prove that the co-administration of Vit E and Ω-3 could pull out the improving effects against DOX-induced hepatopathy. The protection managed by Ω-3 and Vit E might happen via antioxidant-producing enzymes by the cytokines. The produced antioxidants can invert hepatic lesions. Our findings suggest that the proper dose of Vit E and Ω-3 can have a protective complex for anticancer treatment. Vitamin E and Omega-3 are well-known compounds, whose anti-radical and anti-cancer effects were known, but combining them and observing the effects of anti-hepatotoxicity had not been done, which was tried in this research considering the possibilities. In the literature review, it can be seen that the compounds are prescribed in nano form or at least with nano-size transporters. It is suggested to pay attention to the nano composition of omega and vitamin in the field of recent research. It might reduce the cytotoxicity of doxorubicin better and in more detail.
4.1. Conclusions
It has been confirmed that Ω-3 or Vit E separately is an antioxidant with hepatoprotective potential and approaches the combination in a better condition.