1. Background
Breast cancer (BC) is the second most common and the fifth cause of death among various types of cancer worldwide (1). BC is the most frequently diagnosing cancer in women and is one of the causes of cancer mortality. . Metastasis or the spread of tumor cells throughout the body is the leading cause of BC-related deaths (2). Approximately 10% of patients with newly diagnosed BC present with concurrent metastatic disease. Surgery has an important role in diagnosis and staging of BC cases and managing solid organ tumors such as BC, as the patient can be completely cured via definitive resection (3, 4). In addition, it also remains the first-line treatment for a large number of patients (4). Nevertheless, metastasis after the surgery is still frustratingly frequent (3).
The pre-operative and also operative management may likely have effects on the long-term prognosis of BC patients (5). Analgesic drugs and opioids such as morphine, fentanyl, and remifentanil are administrated during and after surgery, as well as during the post-operative period of cancers to relieve chronic pain (6). The administration of opioids and analgesics during the post-operative period of patients with cancer is controversial. A relationship has been reported between the consumption of higher doses of opioids during the initial 3 days after the operation and an increase in the recurrence rate of lung cancer within 5 years (7). Yamamizu et al. reported that opioids suppress VEGF signaling, which leads to the inhibition of tumor angiogenesis (8). In contrast, morphine has been demonstrated to promote angiogenesis and microvascular proliferation and inhibit apoptosis (9). It has been hypothesized that the use of some local anesthetics and anesthesia techniques can enhance long-term survival after BC surgery and may influence the pathophysiology of post-operative metastatic spread (4, 10). Direct exposure to local anesthetics such as lidocaine and bupivacaine has been shown to induce apoptosis in BC cell lines (11). Lidocaine infusion has been also demonstrated to have analgesic and anti-inflammatory effects, the latter effect is exerted through up-regulation of pro-inflammatory cytokines, both in-vitro and in-vivo. Fentanyl is regularly administrated for analgesia and sedation in various surgical settings such as BC (12). It has been recently shown that fentanyl infusion increases apoptosis in an animal study (13). According to the previous studies, various factors, such as stress response associated with surgical trauma, type of anesthesia technique, used anesthetic drugs, acute pain, and opioids, have been implicated in the metastatic process. These factors may promote the proliferation of BC cells that are part of the minimal residual disease (2, 14-16).
Although many of the most common anesthetics drugs are frequently used in BC surgery, their effects on the behavior of tumor cells are still unknown and we still do not know whether analgesic choice affects the patients’ outcome or not. Therefore, in this study, the effect of two analgesic drugs, lidocaine and fentanyl, on survival rate and apoptosis of BC cell line in-vitro was investigated and compared.
2. Methods
2.1. Patient Selection
Sixty patients with ASA score I–III that underwent elective BC surgery with the same grade of BC were enrolled. Blood samples were drowned from all of the patients before surgery. In order to induce anesthesia, a standard regimen was given to the patients, which consisted of premedication with intravenous (IV) midazolam 0.02 mg/kg and fentanyl 3 µg/kg, propofol 1 - 1.5 mg/kg to reach the target BIS index (40 - 60). Then atracurium 0.5 mg/kg and lidocaine 1.5 mg/kg were administered just before endotracheal intubation. Total continuous pump infusion (TCI) was used for the IV administration of propofol; it helps titrate the infusion of propofol and was applied to keep the BIS between 40 and 60. Then the patients were randomly divided into 2 groups, intravenous lidocaine (A) and intravenous fentanyl (B). In groups A and B, BC patients received IV infusion of Lidocaine 120 mg/hour or fentanyl 2 µg/kg/hour, respectively.
The BIS index was evaluated in 5-minute intervals and if BIS was less than 40, anesthetic drug infusion was reduced by 20%, while it was increased by 20% when BIS was more than 60. If any patient showed tachycardia with more than 20% increase from its baseline, fentanyl with 1µg/kg dose was intravenously administrated for hemodynamic control, which was repeated after 5 minutes if the patient did not respond, in spite of proper anesthetic depth. At the end of the surgery, all infusion drugs were stopped and the patients were then recovered and extubated. All the surgeries were unilateral mastectomy with sentinel lymph node(s) biopsy or dissection. Blood samples were drowned again from all the patients one hour after surgery.
2.2. BC Cell Culture Process
The MCF7 human primary BC cell line was received from the National Cell Bank, Pasteur Institute, Tehran, Iran. These cells were all grown and maintained in a humidified atmosphere of 5% CO2 at 37°C in Dulbecco’s modified Eagle’s medium (DMEM) high glucose1X PhenolRed Media containing 10% fetal bovine serum, 5 mL L-Glutamin, and 1% penicillin–streptomycin (complete medium), and sub-cultured every 3 - 4 days. Once the amount of the cells reached 85 - 90% confluence in 25 mL flasks, the flasks (n = 32) were divided into 3 groups including control (C), lidocaine (A), and fentanyl (B). As a control group, serum was collected before surgery from each patient. Venous blood was also sampled 1 hour after surgery. Blood samples were centrifuged at 400 g, and the sera were then stored at −20°C. To examine whether in vitro exposure to the sera of patients who had BC surgery and received 2 different analgesic drugs had any effect on proliferation and apoptosis, the cultured BC cell lines were exposed to sera collected before and after surgery on 24, 48, and 72 hours post-culture in 25 mL flasks. In both groups, A and B, the mean percentage of apoptosis index after exposure of the cultured BC cell line to pre- and post-operation patient`s sera at 24, 48, and 72 hours post-culture was examined via flow cytometry using annexin V-FITC, when the flasks reached up to 85% of their confluence. The exposure of pre-operation sera was used as the control group.
2.3. Apoptosis Assay
The mean proportion of apoptotic cells (ACs) was calculated via flow cytometry using a FITC-conjugated annexin-V/propidium iodide (PI) assay kit. In short, the cell suspensions (5 X 105/mL) were washed with ice-cold PBS, re-suspended in 1x binding buffer (100 mL) and were then stained using 5 mL of FITC-conjugated annexin V (10 mg/mL) and 10 mL of PI (50 mg/mL). The cells were incubated in dark for 15 minutes at room temperature after mixing. A total of 400 mL of binding buffer was added, and the cells were analyzed (FACScan, Becton-Dickinson, USA). The BC cells (MCF-7) were separately gated based on their granularity and size on forward scatter versus side scatter plots. Cells stained with both annexin V and PI were considered as being in late apoptosis, while cells stained with annexin-V were counted as being in early apoptosis.
2.4. Cell Viability Assay Using 3-(4,5-dimethylthiazol-2-yl)-2–5-diphenyltetrazolium Bromide (MTT)
MCF-7 cells were seeded at a density of 5 × 104 cells/well in 96-well plates and incubated at 37°C in 5% CO2. On days 1, 2, and 3 post-culture, 40 μL of MTT solution (5 mg/mL) was added to each well and incubated at 37°C for 4 hours. To solubilize the formazan crystals, the culture medium was detached and the crystals were melted in dimethyl sulfoxide and absorbance was assessed at 490 nm using an enzyme-linked immunosorbent assay reader (Expert 96, Asys Hitch, Ec Austria). The trial was repeated 3 times in triplicate.
2.5. Statistical Analysis
SPSS-22 software was used for statistical analysis. After testing for normality of pairwise differences with the Shapiro-Wilk normality test, the pre- and post-surgery effect of anesthetic drugs on the mean percentage of ACs and MTT assay findings at various intervals, were compared between groups and intra-group using one-way ANOVA and also repeated measures ANOVA as needed. A P-value less than 0.05 was considered statistically significant. Mean age, operation time, propofol consumption, surgery size, and ASA score, as well as mean lidocaine and fentanyl consumption were recorded and compared between groups A and B using independent t-test.
3. Results
Demographic data of the 60 patients and the mean propofol consumption in both groups are presented in Table 1. All patients were in the same physical status based on ASA. There was no significant difference between the two groups in terms of age, duration of surgery, surgical size, and propofol consumption (P > 0.05).
| Analgesic | Mean Propofol | Mean Age | Mean Size | Duration/Min |
|---|---|---|---|---|
| Lidocaine | 781.42 ± 386.12 | 47.70 ± 6.25 | 13.10 ± 4.28 | 142.00 ± 64.08 |
| Fentanyl | 609.37 ± 183.68 | 49.70 ± 10.30 | 14.30 ± 7.08 | 98.50 ± 41.90 |
| P value | 0.11 | 0.40 | 0.89 | 0.10 |
Demographic Data of the Patients and the Mean Propofol Consumption in Both Groups a
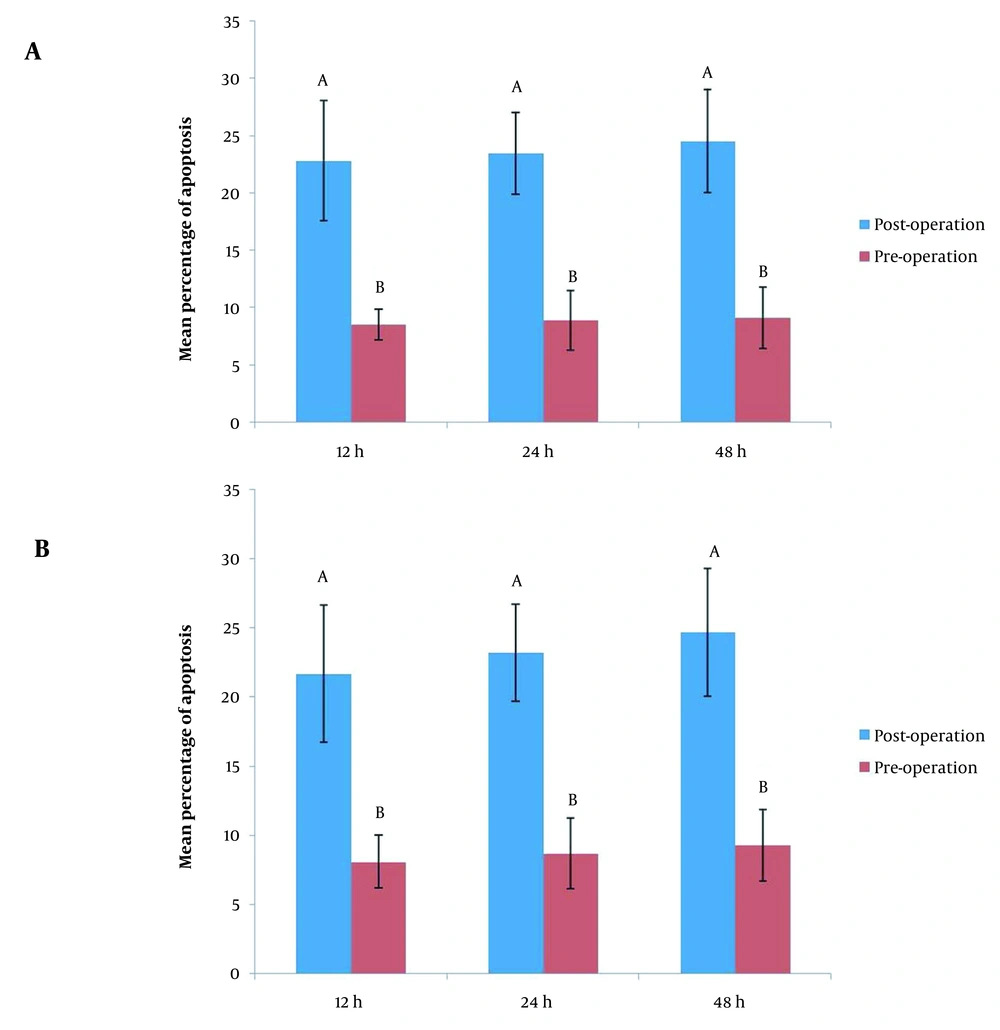
Quantitative cell death analysis showed that the proportion of ACs in group A was 8.50 ± 1.34%, 8.9 ± 2.60, and 9.10 ± 2.70% at 24, 48, and 72 hours post-culture, respectively, when the cells were treated with pre-operation sera of the patients with BC. While the proportion of ACs was 22.80 ± 5.26%, 23.40 ± 3.56, and 24.5 ± 4.50 at 24, 48, and 72 hours post-culture, respectively, when the cells were treated with post-operation sera of the patients with BC.
In group A, intra-group cell death analysis using repeated measure ANOVA revealed that there was no statistically significant difference in exposures of cultured cells to pre-operation sera at various interval times (P < 0.001) with respect to apoptosis index; the same was true for group B (P < 0.001) (Figure 1).
Quantitative cell death analysis showed that the proportion of ACs in group B was 8.10 ± 1.91%, 8.70 ± 2.58, and 9.30 ± 2.58% at 24, 48, and 72 hours post-culture, respectively, when the cells were treated with pre-operation sera of the patients with BC. While the proportion of ACs was 21.70 ± 4.94%, 23.20 ± 3.50, and 24.70 ± 4.60 at 24, 48, and 72 hours post-culture, respectively, when the cells were treated with post-operation sera of the patients with BC (Figure 1).
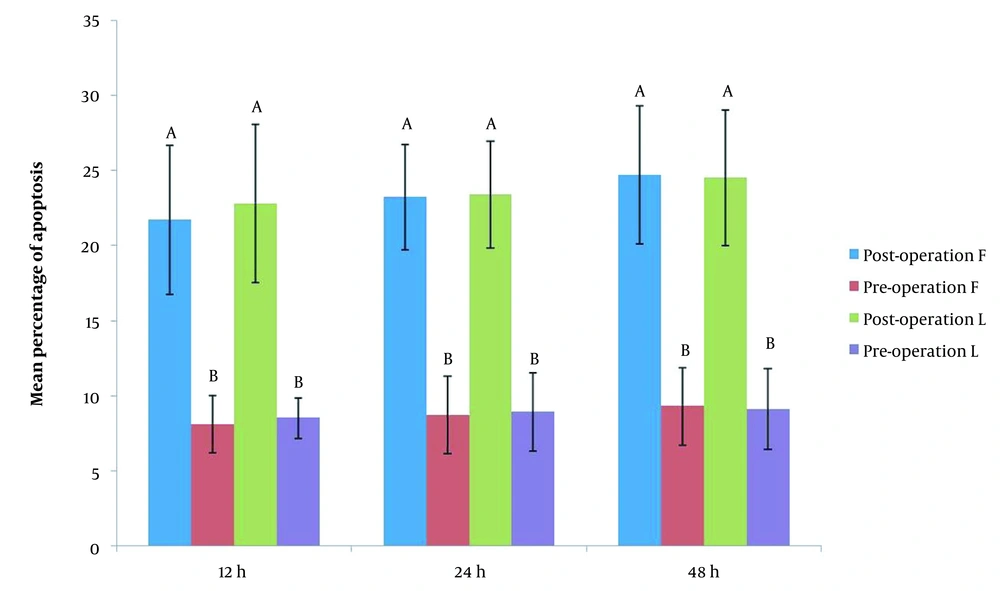
In both groups, intra-group cell death analysis using paired t-test showed that there was a significant difference when cultured cells were exposed to pre-operation sera compared to post-operation sera exposure at various interval times (P < 0.001) with respect to apoptosis index; the same was true for group B (P < 0.001) (Figure 2). However, there was no statistically significant difference between groups A and B with respect to apoptosis index when the cultured cells were exposed to pre- and post-operation sera (P > 0.05).
3.1. MTT Assay
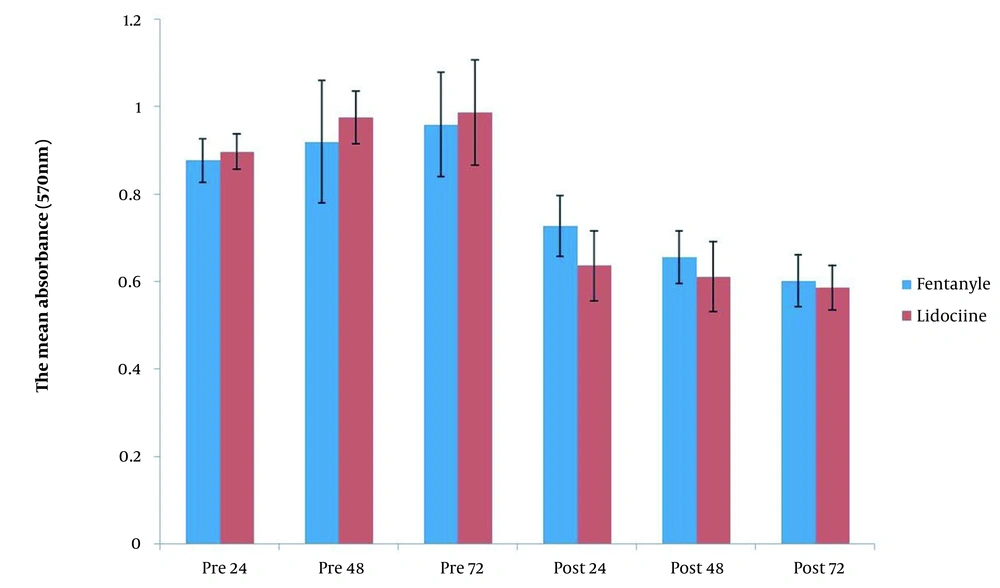
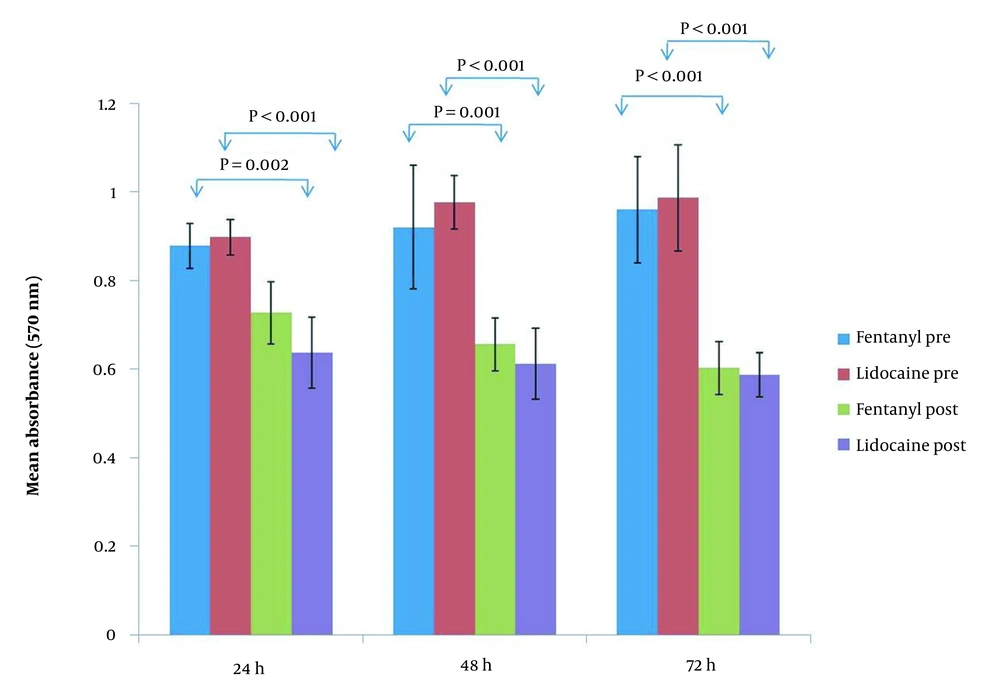
MTT assay was used to show the viability of cultured tumoral cells on days 1, 2, and 3 pre and post-exposure to the patient’s sera. The result of the MTT assay showed that there was no statistically significant difference between tumoral cells on days 1 to 3 pre-exposure in terms of viability before exposure to lidocaine and fentanyl. In contrast, a significant decrease in tumoral cell viability was observed after exposure to both lidocaine and fentanyl compared to pre-exposure on all 3 interval times (Figure 3). In general, there was no statistically significant difference between lidocaine and fentanyl in cell viability before and after exposure at any of the three evaluations at different times (Figure 4).
3.2. Cell Morphology
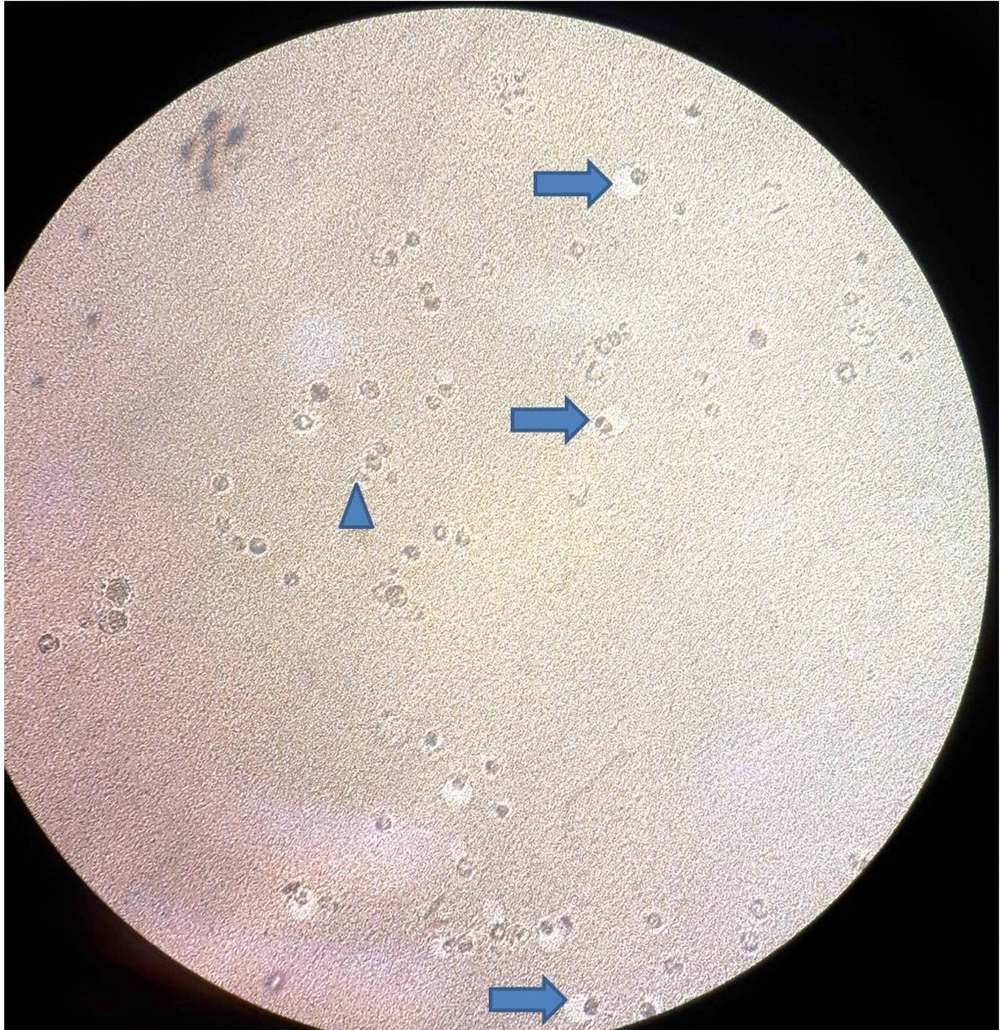
Evaluating the morphology of the cultured BC cells after exposure to the patient’s sera in both groups has shown shrinkage (arrows) with peripheral hallow, isolated from the plate as well as apoptotic bodies (arrowhead) (Figure 5).
5. Discussion
Since the pre-operative period has an important role in the long-term prognosis of BC patients, this study was performed to study the behavior of BC cells in-vitro when exposed to 2 most common administrated analgesic drugs, lidocaine, and fentanyl. We have found that administrating some analgesic drugs, such as lidocaine and fentanyl infusion, in patients undergoing BC surgery can increase the apoptosis of the BC cell line, which also suggests that it may reduce the recurrence of cancer. Although the effect of several pre-operative factors such as type of anesthesia technique and acute stress response during surgical trauma on the post-operation prognosis or the behavior of cancer cells has been suggested (2), the effects of IV infusion of different anesthetics on the behavior of cancer cells are yet unknown. Micro-metastasis or metastatic recurrence after surgery does occur in different cancers and is a major cause of deaths related to cancers such as BC (17). Surgical resection of solid BC is the first and most effective treatment (18); however, metastatic recurrence of cancer resulting from circular micro-metastasis after surgery remains common (3). More than 90% of women in the early stage of BC as well as over 70% of women with advanced BC have undergone breast-conserving surgery (19). Apoptosis plays an important role in metastasis of BC, which has been analyzed in the present study. The data obtained from quantitative cell death analysis showed that after exposure to post-operation sera of patients who received lidocaine, the proportion of ACs at 24 hours post-culture significantly increased compared to cells exposed to the pre-operation sera. A significant increase in apoptosis index was also observed at 48 and 72 hours after culture’s exposure to post-operation sera of patients in this group compared to exposure to their sera before surgery, which shows that some of the factors present in the serum of these patients after surgery promote apoptosis. These findings were confirmed via MTT assay, in which cell viability is identified. The results of the MTT assay showed a higher mean percentage of cell viability after exposure of cultured cells to sera collected before the operation in the lidocaine group. We have the similarly found that in fentanyl group apoptosis index at various time intervals was significantly higher compared to the control group with exposure to patients’ sera sampled before surgery. These findings indicated that both lidocaine and fentanyl can promote apoptosis in cultured BC cell lines. Although we found that the lidocaine group had a higher apoptotic index compared to the fentanyl group, no significant difference was seen between the 2 anesthetic drugs at various times after culture. The findings of this study are in accordance with a previous study conducted by Chang et al. (11). They had demonstrated that cell viability was reduced and apoptosis was induced when breast tumor cells were treated with lidocaine and bupivacaine (11). It has been shown that apoptosis is affected by multiple factors, including immune cytokine signaling (20). A study found that all forms of general anesthesia including intravenous medications, inhalational anesthetics, or most frequently, a combination of both modify the immune system and affect innate and adaptive immunity (21). Anesthetics used in the pre-operative period, can exert an effect on the immune system, directly affect cancer cells, and finally, modify oncological outcomes and prognosis (22). In this period, apoptosis of minimal residual cancer cells resulting from shed tumor cells during surgery, micro-metastatic deposits, and circulating cancer cells could be influenced by multiple factors such as pre-operative immune suppression and the stress response to surgery (23). It has also been shown that the factors affecting the immune system and apoptosis can potentially be modified by anesthetic technique (23).
We found that fentanyl and lidocaine infusion in patients with BC could promote and induce in-vitro apoptosis. The researchers have been wondering if the anesthetic technique affects the prognosis or outcome of cancer in different types of cancer, such as breast, colon, prostate, ovarian, and rectal cancer. Kim has indicated that some techniques of anesthesia can reduce cancer-related mortality and recurrence by inhibiting immunosuppression (24). Despite surgical removal of the primary tumor, chemotherapy, and radiotherapy, 30% to 40% of patients with cancer are estimated to die due to micro-metastasis and cancer recurrence (25).
5.1. Conclusions
The study findings proposed that lidocaine infusion can reach the apoptosis index of BC cells in vitro, as much as that fentanyl did; and both drugs had significant effects. Analgesic choice may likely affect the outcome of BC patients by promoting or inhibiting apoptosis of circular tumoral cells or micro-metastasis.